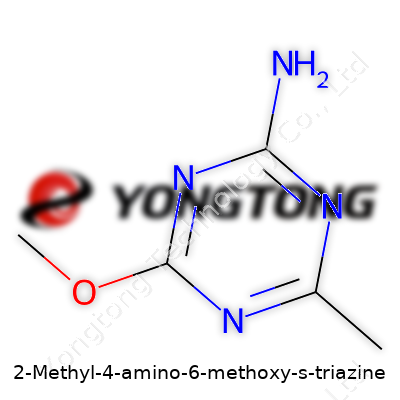
An Article on 2-Methyl-4-amino-6-methoxy-s-triazine
Historical Development
People have tinkered with triazines for decades, building on breakthroughs from the early 20th century. The triazine ring system first drew interest from chemists looking for new therapeutic and agricultural compounds. By the 1950s, researchers pushed hard to create molecules combining nitrogen-rich heterocycles like s-triazines with alkoxy or amino groups, fishing for better selectivity in herbicides and medicines. From there, 2-methyl-4-amino-6-methoxy-s-triazine came into focus as part of rational design. Industry and academia both published reams of articles on better yields, fewer by-products, and clearer analytical structures. The story of this compound ties into global trends, from crop protection right through the quest for fewer side effects in medicinal chemistry.
Product Overview
2-Methyl-4-amino-6-methoxy-s-triazine belongs to a class of compounds often picked for their mix of stability and reactivity. Its triazine core lets it fit into lots of research niches, especially where selective reactivity matters. Chemical suppliers generally offer this compound as a crystalline solid or a fine powder, packaged to guard against moisture and light. Researchers use it for synthetic intermediates and chemical screens, while agronomists value its heritage among triazine herbicides, even as regulations prompt a steady march toward greener alternatives.
Physical & Chemical Properties
This chemical appears as an off-white to pale beige solid. It usually doesn't emit strong odors. Melting points clock in between 150 and 200°C, depending on synthesis conditions and purity. It holds together well under ambient temperatures but reacts with strong acids or bases. Water solubility remains low—an edge in some storage situations, but a headache during reaction cleanups. The methoxy and methyl groups close up some reactive sites, but the amino function holds onto just enough nucleophilicity to open doors for derivatization. Its robust triazine skeleton shrugs off slow heat rises or everyday lab oxygen, but contact with strong oxidizers or flames can trigger decomposition, giving off nitrogen oxides or methane.
Technical Specifications & Labeling
Suppliers selling this molecule mark containers with details essential for traceability: compound name, batch number, purity (often 97% or higher for research use), and hazard warnings based on the current GHS system. Labels mention the chemical and structural formula, UN number (if it's classified for transport), and recommended storage temperature—usually room temperature, shielded from moisture. Certificates of analysis back up each shipment, giving a paper trail for both regulatory compliance and research reproducibility.
Preparation Method
Manufacturers lean on methods developed to maximize both safety and yield. Typical syntheses start with cyanuric chloride, which reacts with a sodium methoxide solution under controlled temperatures, giving 2-methoxy-4,6-dichloro-s-triazine. Next, methylamine swaps in for one of the chlorines, and ammonia or another amine replaces the other. Each step uses stoichiometric ratios tuned for minimal waste and high product purity. Reaction mixtures need close monitoring, since runaway exotherms can cause pressure spikes or side reactions. Workups generally draw from stepwise pH adjustment, careful filtration, and solvent exchange, with recyclable solvents wherever permitted.
Chemical Reactions & Modifications
2-Methyl-4-amino-6-methoxy-s-triazine acts as a versatile building block. The amino group undergoes acylation, sulfonation, and alkylation, expanding its library of analogs. Mild oxidants or electrophilic reagents fine-tune the methoxy or methyl side chains. Chemists can even unmask the triazine core for ring expansion or fusion reactions, feeding demand for denser heteroaromatic scaffolds. Triazine derivatives often show up in structure-activity relationship studies, helping to explore how each chemical tweak influences biological or material properties. Industrial modifications sometimes pursue better water solubility or binding affinity by grafting hydrophilic tails, responding to external regulatory and application-driven demands.
Synonyms & Product Names
This compound pops up in the chemical literature under several alternative names. Chemists might run across it as 2-Methyl-4-amino-6-methoxy-1,3,5-triazine. In agricultural and pharmaceutical documents, product codes sometimes replace full systematic names, shortening it to MAMT or related abbreviations. Legacy trade names still float around, often regional or company-specific, but IUPAC naming rules hold sway in recent research and regulatory circles.
Safety & Operational Standards
Lab and plant workers handle triazines under strict precautions. Gloves and eye protection come standard, along with dust masks if the process generates airborne particles. Handling protocols prioritize closed systems to curb exposure and spills, since triazines in general have sparked debate about persistence and bioaccumulation. Safety data sheets spell out first-aid, firefighting methods (think foam or CO2, not water), and steps for decontamination. Proper waste disposal follows the latest hazardous waste codes, with local customs sometimes ratcheting up – especially in regions with tight pesticide controls. Routine air monitoring fills in any gaps, supported by periodic audits and worker training so that nothing falls through the cracks.
Application Area
Some of the earliest commercial triazines found their way into herbicides, and this continues to shape how new derivatives get assessed. That legacy means 2-Methyl-4-amino-6-methoxy-s-triazine sometimes goes into research on modern crop protection agents. Researchers track its activity against resistant weed strains or compare its environmental footprint to that of classic triazines like atrazine. Formulators eye its chemical stability for specialty coatings, adhesives, and flame retardants, especially where the market demands high-performance materials with tailored degradation profiles. Medicinal chemists occasionally screen it for antimicrobial or antitumor potential, looking for scaffolds both potent and metabolically stable. Even with ongoing regulatory scrutiny, these compounds pull multidisciplinary teams into conversation—from environmental scientists to synthetic chemists and toxicologists.
Research & Development
Research labs around the globe use this chemical to probe new synthetic routes and reaction mechanisms. Development teams focus on greener processing—solvent swaps, better atom economy, and continuous flow methods shave down waste and boost both safety and output. Demand continues from pharmaceutical screens, where triazines pop up as hinge-ligands in kinase inhibitors or as versatile backbones for library synthesis. Academic studies highlight new transformations, sometimes incorporating computational chemistry to map out electron distribution and optimize yields. Competition drives constant improvement, with both public and private labs aiming to beat old bottlenecks in selectivity, scale-up, and cost.
Toxicity Research
Toxicologists find triazines too important to ignore. Studies explore the acute and chronic impacts of exposures, using a mix of in vitro cell lines and in vivo animal models. Data so far show triazine structures range widely in toxicity: some analogs trigger skin and eye irritation, while a few raise red flags for reproductive or developmental endpoints. Regulators demand increasingly rigorous datasets, including endpoints covering bioaccumulation, endocrine disruption, and environmental breakdown. Even small structural tweaks—like swapping one substituent for another—shift results, which keeps both toxicologists and synthetic chemists alert for safer alternatives. Monitoring labs keep environmental water and soil samples under tight review, aiming to catch any signal of persistence early and push for remediation where needed.
Future Prospects
Looking at where the field is headed, pressure mounts for compounds that balance performance and environmental responsibility. Up-and-coming research leans hard into both computational modeling and advanced catalysis, seeking ways to redesign triazines for faster biodegradation and lower human toxicity. Industry voices call for partnerships with regulatory bodies, nudging new triazines into markets only after thorough screening and lifecycle assessments. Materials scientists test out mixtures with biodegradable polymers, ensuring residues don't stick around where they shouldn’t. Triazine chemistry keeps evolving—in both lab notebooks and manufacturing plants—adapting to new safety regulations, shifting market needs, and the ongoing hunt for safer, more sustainable chemicals.
The Unsung Backbone of Many Herbicides
Walking through cornfields during summer, it’s easy to take a green sea of uniform leaves for granted. Here’s the hidden story: behind those neat rows stands a chemical shield, and one ingredient often at play is 2-Methyl-4-amino-6-methoxy-s-triazine. This compound, often called “MAMMT” in chemistry circles, shows up as a building block in some widely used crop protection products, most notably in the synthesis of atrazine and related triazine herbicides. Farmers and field technicians rely on these tools for keeping weeds in check, saving priceless hours of labor and tons of lost crops, especially on big industrial operations.
Atrazine and its relatives, crafted using 2-Methyl-4-amino-6-methoxy-s-triazine, don’t just target any plant. They work by blocking photosynthesis in certain weeds. I’ve watched fields where sections skipped spraying and could see pigweed and foxtail running wild, robbing corn and soybean plants of water and nutrients. This single chemical, wrapped up inside a bag of pellets or liquid concentrate, helps solve a problem that would need endless weeding and, likely, more soil erosion if folks turned to plows instead. It’s not glamorous, but no farmer laughs it off. Losing control over weeds means smaller yields and less food in our stores.
Why Use a Triazine Base?
Some chemists I’ve worked with point out that triazines, including 2-Methyl-4-amino-6-methoxy-s-triazine, really earned their reputation for being reliable in many climates. Their molecular makeup gives manufacturers the flexibility to tweak them, so they stay active in the soil for just the right amount of time—long enough to stop weeds, not so long that cash crops suffer. In the lab, stability matters. Sourcing clean batches and ensuring high purity helps prevent byproducts that could harm non-target plants. So this compound plays two roles, both as a synthetic precursor and as a standard for making sure batches meet tight industry standards.
Health, Risk, and Responsibility
No one who spends seasons working near fields can ignore the debate around chemicals like these. Atrazine faces plenty of scrutiny from health authorities and environmentalists, especially in areas where run-off enters lakes and rivers. There are scientific reports about possible links to hormone disruption in aquatic life and ongoing studies about long-term health impacts for people. That creates a tension between productive farmland and healthy watersheds. I’ve seen local meetings where farmers, researchers, and town officials wrangle over regulations, struggling to balance food security with safety.
One big lesson: knowledge works best in everyone’s hands. Clear labeling, field training on application rates, and regular soil and water testing help reduce unnecessary exposure and limit environmental spread. Researchers have been pressing for better methods—like using cover crops to hold soil, buffer strips near water, and developing weed management strategies that use chemistry only when needed. Companies have responded too, investing in alternatives and cleaner production lines for their triazine compounds.
Looking Ahead to Sustainable Practices
Watching change from the ground up reminds me that 2-Methyl-4-amino-6-methoxy-s-triazine isn’t an evil or miracle on its own. Its value depends on how it’s made, sold, and used. By reminding ourselves of that, growers and industry leaders keep making progress toward safe and effective food production. Supporting continuous review, strong oversight, and newer weed management ideas gives everyone a stake in better agriculture for next spring and beyond.
Getting Conditions Right Isn’t Optional
On the shelves of any lab, every bottle gets a label, but not every chemical gets the attention it deserves. Some compounds will bite you back if ignored. 2-Methyl-4-amino-6-methoxy-s-triazine sits in the category where careful storage isn’t just about ticking boxes—it’s about protecting people, projects, and investment.
Why Storage Demands Attention
Let’s get real: chemical safety isn’t just regulations and paperwork. I’ve seen firsthand how poor storage creates headaches, spoiled compounds, mad dashes to reorder, and rough conversations with auditors. The big issue with triazines, and particularly with this one, rests on their stability and what happens if things go wrong. Moisture, heat, or sunlight can trigger changes—a breakdown of structure, loss of potency, or even side reactions that cause risk in handling.
Key Storage Factors For 2-Methyl-4-amino-6-methoxy-s-triazine
Temperature makes a difference. Most suppliers recommend keeping this compound at room temperature—roughly 20°C to 25°C. Higher heat can kickstart unwanted reactions. A climate-controlled storage room prevents the temperature swings that degrade sensitive molecules. I’ve worked in places that skimp on air conditioning, and you can spot deteriorating compounds by their clumpy texture or weird odors.
Humidity stays enemy number one for many organics. Triazines don’t love water. Sealing containers tightly, using desiccant packs, and storing the chemical in a dry spot should be standard habit, not an afterthought. I remember opening a jar stored near a lab sink, only to find the powder caked and useless, because humidity had crept in and done its damage quietly.
Light exposure brings its own problems. Even though this compound isn’t highly photosensitive, direct sunlight will warm containers and induce gradual changes. Simple cardboard boxes or shaded shelves help. Sometimes labs overdo it with clear glass jars right under fluorescent lights—an easy fix is shifting everything to amber bottles or at least using opaque alternatives.
Ventilation and Compatibility can’t be overlooked. Triazines should never sit next to oxidizers, acids, or bases on a crowded shelf. I’ve seen near-misses where shelf mix-ups sparked hazardous reactions. Keeping the storage spot well-ventilated keeps tiny leaks from turning into big issues.
Staff Knowledge, Labeling, and Inspection
The biggest trouble doesn’t always come from freak accidents, but from everyday habits gone lax. Proper labeling with hazard warnings in plain language helps new team members avoid mix-ups. Staff training means everyone understands what stays where, how to check container seals, and how to spot changes in the powder. Inspection schedules, even once a month, keep surprises at bay. That kind of diligence comes from caring about the people who handle the material, not just about meeting some regulation.
The Bigger Picture: Risk, Waste, and Responsibility
Losing an expensive compound or risking contamination sets projects back, wastes resources, and sometimes puts people in harm’s way. Storing 2-Methyl-4-amino-6-methoxy-s-triazine correctly ties into good science and good stewardship. We work hard to get great results, and nobody wants a failed run at the finish line because of sloppy storage. Keeping conditions right pays off over and over—more than just shelf-life and savings, it’s about keeping the lab safe for everyone who steps through the door.
What Makes This Chemical Stand Out
Chemicals tend to get more attention when they have long, complicated names. With 2-Methyl-4-amino-6-methoxy-s-triazine, you might think this is some cutting-edge compound that belongs in a research lab. The truth is, it’s a triazine—a family of chemicals long used in pesticides, herbicides, and industrial applications.
Looking Up the Safety Data
Before anybody starts worrying or downplaying, it’s important to check hard data. Reliable sources, like Safety Data Sheets (SDS) and the PubChem database held by the National Institutes of Health, give a rundown on the risks. According to published documents, 2-Methyl-4-amino-6-methoxy-s-triazine can cause eye and skin irritation. Breathing in its dust is linked to respiratory tract irritation—a red flag for anyone working with raw forms in a lab or factory.
Now, skin or eye irritation doesn’t sound dramatic, but the story doesn’t end there. Chemicals that irritate on contact sometimes signal bigger risks below the surface. If proper handling is skipped, unexpected exposure can add up, turning simple discomfort into chronic problems. Having once spent time working near industrial solvents, I’ve seen folks brush off “just irritation”—until it starts affecting daily life.
Toxicity and Human Health
So far, the research on 2-Methyl-4-amino-6-methoxy-s-triazine’s chronic toxicity in people hasn’t grabbed many headlines. Still, this doesn’t hand out a free pass. Close cousins in the triazine family, such as atrazine, have landed under the spotlight for possible links to hormone disruption and environmental toxicity. Given the similarity in structure, scientists keep an eye on these related compounds.
Most available data focus on animal testing and cell studies, hinting at toxicity if large amounts wind up inside a living organism. Occupational guidelines, where they exist, set exposure limits designed to dial down that risk. The U.S. EPA, for other triazines, recommends strict monitoring and control measures, especially if chemicals could contaminate drinking water or soil.
Protecting Workers and Communities
On site, responsible employers fit out their teams with gloves, goggles, face shields, and ventilation systems. This isn’t just about ticking a box—these steps keep people from breathing in fine dust or splashing solutions on the skin. Training matters, too. Having clear instructions and easy-to-read labels can mean the difference between quick action and hesitation in an emergency.
Beyond the fence, chemicals like 2-Methyl-4-amino-6-methoxy-s-triazine deserve careful handling. Safety at the plant spills into the neighborhood. Once, at a nearby facility, a minor leak led to complaints about foul air. Clear communication, quick mitigation, and transparent testing kept the community’s trust—but it took planning ahead, not wishful thinking.
What Comes Next
Nobody should jump to panic or assume safety without proof. Industry and regulators depend on ongoing research, honest risk assessment, and updates whenever new findings emerge. That’s the lesson from decades of both progress and mistakes in chemical manufacturing.
Bottom line: treat unknowns with respect, follow precautions, and push for better science. My own experience with chemical safety boils down to a simple idea—better to take a minute and avoid trouble than spend weeks fixing it. Triazines deserve that kind of attention.
Diving into the Formula and Importance
2-Methyl-4-amino-6-methoxy-s-triazine, a chemical name that doesn't roll off the tongue, actually tells a detailed story through its formula: C5H8N4O. Each part reveals the makeup of this compound: five carbon atoms, eight hydrogens, four nitrogens, and one oxygen atom. Sorted by molecular weight, this compound tips the scale at 140.14 g/mol.
Getting a Grip on the Chemistry
This isn't just another number in a chemical database. 2-Methyl-4-amino-6-methoxy-s-triazine stands as a building block in pharmaceutical and agricultural research. The triazine core boasts strong relevance thanks to its roles in herbicide development, particularly in products that keep pests away and food production up. Through the aromatic ring—with its three nitrogens—chemists create molecules meant to handle real-world challenges.
Why Details in Formulas Matter
Chemical formulas look cryptic until you think about their job. I worked in an agricultural research team that often struggled with pest resistance. Understanding the fine points of each molecule wasn’t a trivia pursuit; it played a role in creating treatments that balanced strength with safety. 2-Methyl-4-amino-6-methoxy-s-triazine, with specific molecular tweaks, often made the difference between a targeted effect and an environmental problem.
Real Impacts and Challenges
Companies keep seeking molecules that pack enough punch to protect crops or fight disease but leave minimal marks on the environment. Healthcare teams face mounting roadblocks with resistant infections, which put a new lens on these foundational chemicals. If research dives deep into something like 2-Methyl-4-amino-6-methoxy-s-triazine, the focus isn’t on the whole world of triazines, but on the details—each methyl, amino, and methoxy group changes how the material interacts with water, soil, or living tissues.
Evaluating the Path Forward
Mistakes in formula identification waste time and money—and may even slow the regulatory process. Trustworthy, double-checked records on molecular weights and formulas support the work for safe product launches. Data on C5H8N4O can’t get fudged or left unchecked; experiments often run on narrow margins, and an error in the molecular weight calculation might create a domino effect.
Better Solutions Through Trust and Openness
Many labs now use open-access databases and publish findings with peer review front and center. Credibility rises when researchers cite reliable sources and show data trails. Regulatory agencies and consumers both want transparency on what’s used in fields and pharmaceuticals. Bringing chemists, environmental scientists, and health experts together sharpens the discussion. Their combined perspectives give society a better shot at solving pesticide resistance or contamination puzzles that triazine-related chemicals sometimes bring.
Grounded Data Drives Safe Innovation
I’ve seen small mistakes in chemical data ripple out, affecting everything from raw ingredient sourcing to final approvals. The weight and formula for chemicals such as 2-Methyl-4-amino-6-methoxy-s-triazine serve not just as identifiers, but as the backbone for responsible science and global safety.
Understanding the Risks Behind the Name
Anyone who has fiddled around with organic chemicals, especially in research or industrial spaces, knows the name 2-Methyl-4-amino-6-methoxy-s-triazine often rings a bell for its role in making herbicides. It doesn’t take a chemical engineering degree to respect these substances. Dust particles, liquid splashes, or unpredictable spills all carry potential hazards. I’ve had more than a few close calls in the lab, and the chemistry textbooks rarely show just how quickly a casual attitude translates into accidents. For this one, direct skin or eye contact, inhalation, or even accidental ingestion can cause irritation or more stubborn health effects.
PPE Isn’t Just for Show
Chemical-resistant gloves, lab coats, and splash goggles quickly become second nature. Just slipping on nitrile gloves keeps skin away from surprises. Some older labs still use latex, but I saw too many reactions between latex and solvents, so the switch to nitrile brought peace of mind. Well-fitted goggles cut down the temptation to rub tired eyes mid-work. If dust threatens to hang in the air, a particulate respirator goes on. Nobody enjoys mask lines on their face, but tradeoffs like these are simple compared to a nasty cough or worse.
Ventilation Makes All the Difference
A stuffy workspace lulls people into cutting corners. When working with chemicals with even a hint of volatility, a solid fume hood isn’t optional. Decades of studies show lower exposure levels and reduced long-term complications in people using proper airflow. I still remember one lab inspection where an old hood sputtered along—most students didn’t even know it needed maintenance until someone started coughing. Simple fix: check your hood’s airflow with a bit of tissue or a test strip before starting.
Don’t Just Read Labels—Understand Them
Safety data sheets hold a reputation as tedious paperwork, but ignoring these details borders on careless. Key hazards show up right on the front. For 2-Methyl-4-amino-6-methoxy-s-triazine, warnings about eyes and skin pop up, but the real nuggets are in the handling and storage sections. I still keep hard copies around, marked up with highlighters where first aid advice appears, because in emergencies, hunting around digitally wastes precious seconds.
Spill Response Isn’t Just Mopping Up
A minor spill gets swift, methodical attention. Absorbent pads, a little sodium bicarbonate, and a scoop set live in my spill kit drawer. Plenty of staff forget the importance of working methodically—no need to sprint around and spread dust into places it doesn’t belong. Containment commands the first step, then cleanup, then a full soap-and-water wipe down of nearby surfaces.
Waste Disposal and Good Habits
Tossing used gloves or powder into the regular trash can creates issues far beyond just your own bench. Designated waste bins, labeled by hazard class, stand ready in most workspaces, but forced habit makes the difference. Regulatory trouble often starts with one misstep in the waste log, and the headaches that follow cut into productivity and peace of mind.
Training and Reinforcement
One-off safety briefings never stick. Routine spot checks and practical drills, much like fire alarms, ensure that old reminders stay fresh. I’ve found teaching new colleagues the “why” behind each step—rather than just barking orders—drives home both respect and personal investment.
Takeaways: Vigilance and Respect
Safe handling comes down to real habits, reliable equipment, and a healthy respect for both the substance and people involved. Companies and labs already have rules, but it’s the front-line attention paid each day that keeps fingers, eyesight, and breathing unscathed.