2-Fluoronitrobenzene: An Analytical Commentary
Historical Development
The journey of 2-Fluoronitrobenzene runs parallel to the growth of fluorinated aromatic chemistry, tracing back to the surge of synthetic methods in the mid-20th century. As researchers aimed for greater precision and potency in pharmaceuticals and agrochemicals, the introduction of a fluorine atom onto the nitrobenzene backbone became more than an academic exercise. Crews in European and American laboratories leaned into the selective substitution techniques, which had to balance hazardous conditions with the drive for innovation. This approach allowed even small-scale facilities to synthesize complex structures, setting the stage for widespread applications and broader accessibility in research and manufacturing.
Product Overview
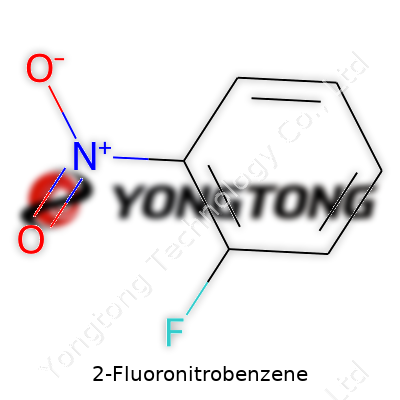
2-Fluoronitrobenzene often finds a place on the crowded shelf of building blocks, standing out for its reactive fluoro and nitro groups. The molecule, consisting of a benzene ring bearing one fluorine and one nitro at ortho-positions, allows for further chemical tweaking without falling apart under pressure. Its yellow crystalline form makes manual handling straightforward in most academic and industrial settings. Manufacturers offer this compound in a range of purities, usually hitting 98% or above, which speaks to the market’s demand for consistent quality during synthesis.
Physical & Chemical Properties
The physical makeup matters. With a melting point around 21°C and boiling point near 210°C, 2-Fluoronitrobenzene straddles the line between solid and liquid at room temperature, creating flexibility for storerooms and labs. Its modest vapor pressure brings less risk of inhalation at ambient conditions, but it won’t evaporate away quickly during a spill. The molecule’s solubility in organic solvents such as diethyl ether or ethanol reflects its origins and uses, and its slightly sweet, sharp odor hints at the reactivity packed inside. The electron-withdrawing nitro and fluoro groups shift chemical behavior, amping up electrophilic substitution possibilities.
Technical Specifications & Labeling
On a product sheet, the technical language peels back to essentials: purity, water content, isomeric ratio, and packaging. Reputable suppliers print a lot number, batch details, hazard pictograms, and UN number for transparency from warehouse to waste stream. Based on GHS labeling, the compound earns pictograms flags for both acute toxicity and environmental hazard, given its nitro and fluoro combo. Authenticity stands out through rigorous spectroscopy data, matching against NMR, IR, and mass spectra. Manufacturers also list shelf life, often remaining stable for two years if kept in a dry, cool environment away from sunlight and incompatible chemicals.
Preparation Method
Classical synthesis often starts from nitrobenzene derivatives, relying on halogenation steps toughened by careful controls. A handful of routes exist, but the Balz–Schiemann reaction—or direct fluorination using milder conditions—has grown in popularity thanks to greater selectivity and improved yields. In practice, some processors take advantage of copper-mediated halogen exchange, starting with 2-chloronitrobenzene and swapping in fluoride. The tradeoff—handling potentially toxic reactants for the sake of a cleaner substitution—pushes engineers to invest in extraction hoods, PPE, and robust waste neutralization.
Chemical Reactions & Modifications
Given its structure, 2-Fluoronitrobenzene opens the door for nucleophilic aromatic substitution, especially as the nitro group activates the ortho and para positions relative to fluorine. It serves as a launching point for further derivatization in both academia and industry, whether that’s moving toward aniline products by reduction or coupling to complex heterocycles needed in pharmaceuticals. The compound embraces aminolysis, hydroxylation, and hydrodehalogenation, reacting with nucleophiles like amines, thiols, and hydroxide ions without demanding extreme conditions. The reliability of its chemistry has cemented its spot in multistep syntheses for dyes, herbicides, and drug candidates.
Synonyms & Product Names
The chemical goes by several names, each cropping up in catalogs or literature: o-Fluoronitrobenzene, 2-nitrofluorobenzene, and 1-fluoro-2-nitrobenzene. No matter the term, the characteristic pair of substituents—fluorine at the ortho spot to a nitro group—ties them together. Some suppliers tag product numbers or custom codes, but those rarely carry outside one firm’s internal system. Recognizing these alternate names makes literature searches and cross-lab collaboration much smoother.
Safety & Operational Standards
Handling 2-Fluoronitrobenzene demands respect for hazardous chemical protocols. Exposure brings risks through both skin and inhalation, potentially causing headaches, dizziness, and respiratory issues. Chemical safety experts advise donning nitrile gloves and splash goggles, and sticking to chemical fume hoods during mixing or weighing. Emergency response plans cover both small spills—managed with inert absorbents—and larger events requiring full-scale decontamination. Disposal follows rules for halogenated organic wastes, never poured down drains or handled with bare hands. Regulatory standards—OSHA, REACH, and the EPA—guide storage, labeling, and employee education, with regular audits needed in high-throughput facilities.
Application Area
2-Fluoronitrobenzene finds heavy use as an intermediate for pharmaceuticals, agrochemicals, and specialty materials. Synthesis of anti-infective drugs and herbicidal compounds lean on this building block to anchor novel structures or introduce metabolic stability. Coatings and polymer modifiers also benefit, where both softness and chemical resilience count. Its value shows up in projects moving from milligram-scale in academic labs to multi-ton production in industrial parks—demonstrating both flexibility and staying power. It supports both custom small-batch work and mainstream production lines, connecting chemical research to hands-on applications.
Research & Development
R&D teams regularly experiment with ways to widen the usability of 2-Fluoronitrobenzene. Advances in synthetic methods target greener, safer pathways, cutting the use of harsh halogen sources or swapping out dangerous solvents for water-based blends. Chemists test its reactivity in new cross-coupling reactions, aiming to lower costs or expand the scope of products downstream. Data flow from bench experiments into patent filings, academic publications, and industrial process revamps, making the molecule a touchstone for testing the frontiers of aromatic chemistry and sustainable synthesis.
Toxicity Research
Toxicologists have spent years pinning down the risks tied to 2-Fluoronitrobenzene. Short-term exposure studies show central nervous system effects in rodents, and some derivatives cause cellular stress or DNA damage in vitro. Human data remain limited, pushing regulators to recommend the highest possible control measures. Chronic studies seek to answer how the compound impacts metabolism in mammals, its breakdown products, and the risk from repeated contact in manufacturing settings. Environmental research watches for toxic run-off, aiming to stay ahead of regulations tied to fluorinated organics which build up in soil and waterways.
Future Prospects
Looking ahead, the future for 2-Fluoronitrobenzene remains tied to the push for safer, more sustainable chemistry. Researchers continue to seek out alternative synthesis routes that leave a lighter footprint on the environment, especially as demand for fluorinated pharmaceuticals and agricultural products grows. Digital technologies—AI-driven reaction optimization or remote process monitoring—promise to slash waste and improve yields. Expansion into new fields like organic electronics or cleaner energy materials could reshape the markets and foster tighter safety standards. As scientists learn more through targeted toxicology and biodegradation work, ongoing dialogue between industry, regulators, and environmental groups will determine how this class of compounds fits into a changing chemical world.
Digging Into Everyday Chemistry
If you’ve ever glanced at the ingredients behind everyday pharmaceuticals, cleaners, or modern tech, you’ve come across names that seem like tongue twisters straight out of a chemistry textbook. One of those is 2-Fluoronitrobenzene. The name probably doesn’t stir much excitement unless you’ve set foot in a lab or pharmaceutical plant, but its footprint stretches further than most of us realize.
Why 2-Fluoronitrobenzene Matters
Years in research taught me that chemistry works like a domino effect — one change in a simple molecule often sparks bigger breakthroughs. 2-Fluoronitrobenzene carries both a nitro group and a fluorine atom attached to a benzene ring. On paper, that seems simple. In practice, that combo gives chemists a flexible tool for building advanced materials and drugs.
The fluorine atom stands out. It can transform a drug's behavior, improving its performance or helping it slip past the body’s defenses better than similar drugs. The nitro group isn’t just window dressing — it acts like a chemical handle, letting chemists swap, add, or tweak linked parts to tailor new molecules.
The Heart of Pharmaceutical Research
Turn back a few years and you’ll notice drug development exploded with the help of fluorinated compounds. Drugs with a fluorine atom sometimes last longer in the body or act more potently, so they need smaller doses. Medications for depression, cancer, and even high cholesterol use this trick. Synthesizing fluorinated molecules from scratch can test any chemist’s patience, though, and that’s where 2-Fluoronitrobenzene comes into play. It’s a favored ingredient for building these more complicated structures without time-consuming detours.
Researchers often start with 2-Fluoronitrobenzene, then snap on new molecular parts. That way, they can test new treatments rapidly. With strict regulations steering drug development, labs need to repeat and refine their experiments. Reliable building blocks, such as this one, help keep innovation moving fast and smooth.
Building Blocks for Industries Beyond Medicine
Now and then, a molecule finds work on more than one stage. The electronics world chases after materials that can survive heat, static, and heavy use. 2-Fluoronitrobenzene sometimes feeds into the production of specialty polymers or advanced dyes for displays. It shows up in colorants that resist fading and materials designed to keep functioning under tough conditions.
Agricultural science also taps into this chemistry. Pesticides and herbicides demand molecules that stick around long enough to work but break down before sticking around forever. Fluorinated nitrobenzenes help adjust this balance for better crop protection with fewer long-term risks.
Meeting the Responsibilities That Come With Power
Chemicals like 2-Fluoronitrobenzene carry power. They promise progress in healthcare, electronics, and farming. They also ask us to take safety seriously. Fluorinated compounds sometimes linger in nature, so recycling and proper disposal matter as much as the original discovery. Factories and labs need solid safety protocols and investment in greener chemistry. Cutting waste and developing new reactions can lighten the impact on the environment.
Better methods for recycling chemicals or designing ones that break down safely in the wild aren’t just wishful thinking — they shape the kind of world we pass on. As researchers seek safer alternatives and production methods, 2-Fluoronitrobenzene keeps reminding us that every advancement comes with the duty to work responsibly.
Understanding the Risks in the Lab
Working in a chemistry lab, I’ve seen my share of reactive chemicals. 2-Fluoronitrobenzene stands out. Its sharp odor tells you it isn’t just another bottle on the shelf. Toxic dust and vapors create an environment where simple mistakes turn costly fast. The nitro group means this compound brings both toxicity and reactivity into the mix. A colleague once overlooked a tiny leak during weighing — a day of headaches and lab downtime taught us nobody gets to feel casual around this stuff.
Personal Protective Equipment: Suit Up or Pay the Price
Cotton lab coats never cut it with 2-Fluoronitrobenzene. I always reach for a chemical-resistant apron, doubled with sleeves that don’t pull up. Nitrile gloves give enough protection, but swapping out gloves at the first sign of damage should become automatic. Goggles don’t just hang around my neck — they stay on my face, blocking out splash and vapor alike. Face shields come in handy during larger transfers. Nothing feels more secure than going home with skin untouched and eyes unburnt.
Ventilation Matters
Every chemist hears stories about forgotten fume hoods. Ventilation saves you every single time with volatile organics. I keep every transfer, reaction, and measurement under a running hood. Portable extraction arms help during transport between rooms, especially when the main hoods fill up. Poor airflow means vapor headaches — and those don’t just ruin productivity. Long-term exposure damages nervous systems, and studies link such compounds to thyroid issues. Swapping jokes with a safety officer beats filling out incident paperwork any day.
Storage Isn’t Just an Afterthought
Storing 2-Fluoronitrobenzene right makes all the difference. Locking it away in a chemical-proof cabinet, clearly labeled, reduces the chance of accidental grabs. Temperature control keeps the compound stable. I look for containers without cracks, screw threads in good shape, and tightly sealed lids. Flammable-liquid cabinets offer a good balance by keeping temperatures consistent and breakage risks low.
Spills and Disposal: Be Ready Before Trouble Starts
Minor leaks aren’t rare, especially with old containers. I keep absorbent pads and chemical neutralizers lined up in spill kits. Quick response stops vapors from filling the lab and keeps minor problems from turning into evacuations. Used material gets double-bagged and sorted for hazardous-waste pickup. Dumping solvents or contaminated pads in the regular trash is never an option. I’ve met local environmental compliance officers; fines and mandatory shutdowns follow poor disposal practices. Regulations exist for a reason — spent chemicals have found their way into waterways, causing serious local health problems.
Training and Shared Responsibility
Everybody in the lab has an obligation to speak up. I keep posters up near sinks and hoods: reminders make safety more approachable. Real-world experience counts, but new staff need hazard walkthroughs and rescue drills. This is not about fear or rules — a day’s work shouldn’t result in coughing fits, irritated skin, or worse. Manufacturers provide detailed safety data sheets for good reason. I read them, keep printed versions handy, and digital copies bookmarked. Decisions in the moment save time, money, and health.
Staying safe with something like 2-Fluoronitrobenzene requires good habits, common sense, and real respect for the risks. No shortcut or half measure covers the cost of a single exposure. Real people face the consequences, so focus, real gear, and informed choices are always worth it.
Chemical Formula of 2-Fluoronitrobenzene
The formula for 2-Fluoronitrobenzene is C6H4FNO2. Breaking it down, this means the compound has six carbon atoms, four hydrogens, one fluorine, one nitrogen, and two oxygens. The precise arrangement matters because putting that fluorine in the ortho position next to the nitro group changes how the molecule reacts. This little swap can create a world of difference when someone uses it as a building block for larger or more complex compounds.
Molecular Weight Matters
The molecular weight of 2-Fluoronitrobenzene is 141.1 g/mol. It’s easy to overlook how much weight shifts with just a single atom like fluorine replacing a hydrogen on a benzene ring. That change in weight and the added electronegativity from fluorine combine to tweak the way the molecule interacts with others. Chemists turn to numbers like these every day, not just in labs but across pharmaceutical, agricultural, and industrial applications. One miscalculation can send a whole synthesis off track.
Real-World Uses and Safety Concerns
Plenty of research labs across the globe rely on 2-Fluoronitrobenzene as an intermediate, especially when developing new drugs or specialty chemicals. Fluoro-substituted nitrobenzenes have paved the way for new antifungal agents, anti-inflammatory drugs, and advanced dyes. The versatility in its chemistry keeps the compound in demand. Yet, most people outside science circles rarely think about what rolls down the pipeline from these basic chemicals. Stories about breakthroughs gloss over how much care goes into understanding what goes into these molecules, not just what comes out.
Anyone handling 2-Fluoronitrobenzene needs to respect its properties. It doesn’t pose as much risk as some highly toxic industrial chemicals, but skin and eye irritation, or even respiratory issues, can surface with improper handling. Strong safety protocols and education about handling aromatic compounds should never take a back seat, especially for students and early-career researchers. The real risk grows when inexperienced hands ignore gloves, fume hoods, or ventilation systems, thinking something as simple as a substituted benzene won’t cause trouble.
Improving Accessibility and Stewardship
Chemical information needs to be accurate and available, not only for researchers in elite labs but also for students or makers who tinker with synthesis or small-scale innovation. Mistakes in formulas or weights lead to botched experiments, wasted resources, and sometimes danger. Open sharing of data through reputable sources and regular updates as regulations shift help keep the field honest. For these reasons, I always double-check with trusted databases and cross-reference when it matters. Academic teams, open-source scientists, and startups all make better progress when they use consistent, reliable data about basic chemicals like 2-Fluoronitrobenzene.
Supporting responsible science means remembering that even time-tested molecules require current knowledge. Whether making a new material for greener electronics or adding a new reaction to the undergraduate curriculum, accuracy and safety go hand in hand. Scientific progress isn't just about what we build; it’s also about how carefully and thoughtfully we do it, right down to the small details on a molecular formula.
Why Proper Storage Matters
Every lab worker remembers their first intake safety briefing—a few rules burned in memory, others learned the hard way after a long cleanup. Storing chemicals like 2-Fluoronitrobenzene always sits on that list for a reason. This compound, sharp and persistent, belongs with other nitrobenzenes in terms of risk. A quick glance at its hazard profile will show an orange warning triangle: toxic if inhaled, harmful to organs, irritating to skin and eyes. It isn’t just regulatory box-ticking—one missed step could lead to an emergency or an expensive fume hood overhaul.
Avoiding Trouble with Reactive Compounds
In my own experience, accidents rarely look dramatic. A forgotten solvent, an unlabeled bottle, temperatures just a little too high for a summer weekend—gradually, something leaks or decomposes. 2-Fluoronitrobenzene is stable, but reacts badly with bases, strong reducing agents, and strong acids. Keeping it away from these chemicals makes the day smoother, and gives everyone in the lab peace of mind. I always kept a separate shelf for compounds like this, and more than once, that practice kept a small spill from becoming a bigger problem.
The Right Container and Workspace
Recommendations tend toward the obvious because, in chemistry, obvious steps save hands and lungs. Store 2-Fluoronitrobenzene in tightly sealed glass containers. Polyethylene and polypropylene work too. I learned the hard way that cheap plastic deforms over time and nobody likes sticky residue all over the shelf. Label every bottle clearly with both the chemical name and a hazard symbol—permanent markers, not Post-it notes. Even experienced lab techs grab the wrong bottle on busy days, so direct labeling keeps confusion to a minimum.
Temperature and Ventilation
Temperature means more than comfort—anything too warm speeds up volatilization or decomposition. Room temperature works, but avoid bright sunlight and radiators. Heat rises near windows; a cool, dark cabinet does better. Good air circulation prevents harmful vapor buildup. At my last job, fans never worked as hard as they did in the prep room for aromatic compounds. Store 2-Fluoronitrobenzene in a flammable cabinet fitted with local exhaust ventilation. One slip, maybe a poorly sealed bottle, and the place smells like a burnt bitter almond, which signals trouble fast.
Separate from Food and Personal Items
Labs and kitchens don’t mix, and yet I’ve seen lunches stored next to solvent bottles. Keep all food and drink out of chemical areas. Don’t risk cross-contamination from careless storage choices. Night shifts can blur boundaries, but there’s no excuse for letting workspace hygiene slip. It’s not just about rules—repeated low-level exposure to nitroaromatics brings real health consequences, including anemia and liver problems over time.
Planning for Spills and Emergencies
Even with perfect storage, spills happen—somebody bumps a shelf, a cap breaks, or a label peels off. Keep spill kits with absorbent pads close by and know where the nearest eyewash and emergency shower are. Safety training isn’t a box to check once a year. Regular refreshers stick. Every chemist, from undergrad to veteran, benefits from muscle memory during a real emergency.
Conclusion: A Little Diligence Goes a Long Way
Chemical safety relies on habits, not just policy binders. I’ve seen smart people get careless and spend weeks dealing with the fallout. Store 2-Fluoronitrobenzene with respect and in proper conditions. The result: fewer close calls, less wasted material, and a safer place to work for everyone.
A Look at How This Compound Shows Up in the Lab
Pulling a bottle of 2-Fluoronitrobenzene off the shelf, I remember how unmistakable it is. The pale yellow liquid inside gives off a noticeable, pungent odor—enough to stick in your mind long after you've put the cap on tight. It isn’t some mystery solvent lost in a sea of colorless chemicals. That odd whiff reminds you just how careful you need to be.
The compound soaks up attention partly because of its physical quirks. With a molecular weight just north of 140 grams per mole, it doesn’t feel particularly heavy for an aromatic nitro compound. The melting point isn’t worth bragging about either. Unless your freezer dips below –20 °C, the bottle’s contents stay liquid, waiting for a cold spell to surprise you with some crystals along the neck. The boiling point lands at about 215 °C. That gives it some staying power when the heat’s on, making distillation pretty easy, as long as you're not getting sloppy.
Density and Solubility: What You Really Deal With
Density hovers around 1.38 grams per cubic centimeter. This sinks fast in water but not by much compared to other aromatic compounds in the same family. Still, it won’t float, which always helps with clean-up. Water solubility sits way down the chart. You can't dump this in the sink after an experiment and hope things work out. What ends up in the drain stays in the pipes. It dissolves better in typical organic solvents—ethers, chlorinated solvents, and most things you’d actually want to use in a synthesis. That’s a mixed blessing in the chem lab, easier extractions but also more work if you spill something.
How These Properties Matter on the Job
Chemists look for patterns. 2-Fluoronitrobenzene brings two big features: fluorine locked onto the ring and a nitro group pulling electrons in another direction. Those changes show up everywhere: the odor, the color, the solubility, even the way it heats up in your hands. That pungent smell I mentioned isn’t a joke—it comes from volatile nitro-aromatics, which are almost always more intense than regular aromatics. It's one part warning and one part fingerprint.
Working with this stuff takes some respect for its reactivity and its hazards. It’s not just the low flash point that should keep folks alert. Nitrobenzene derivatives are notorious for being tough on the body if you breathe too much vapor or get it on your skin. Adding a fluorine atom doesn’t make it any friendlier. In my own experience, a fume hood isn’t optional; nothing ruins your day quicker than inhaling a cloud while pipetting under the open air. Even if you’re used to handling bigger hazards, complacency with these compounds invites trouble.
Why Facts Matter: Safe Use and Environmental Impact
Quick clean-up runs smoother thanks to a liquid at room temperature, but the spill risk remains real. The density means it pools rather than skitters across glassware, which sounds trivial until you're dealing with broken vials. Waste handling teams caution against dumping it anywhere watershed can reach. Plenty of research suggests nitrobenzenes, especially halogenated ones, hang around in water and soil, slowly breaking down into nasty byproducts. That long decomposition makes containment and safe disposal even more critical, especially outside of academic labs where oversight might vary.
There’s always talk about safer alternatives and greener chemistry. But as long as synthesis relies on building blocks like 2-Fluoronitrobenzene, nobody can afford to ignore these physical properties. Storage protocols, personal safety habits, and waste treatment plans all tie back to the details—the density, volatility, and the chemical stubbornness of this sharp-smelling liquid. Knowledge turns into practice only when you feel the risks and quirks firsthand.