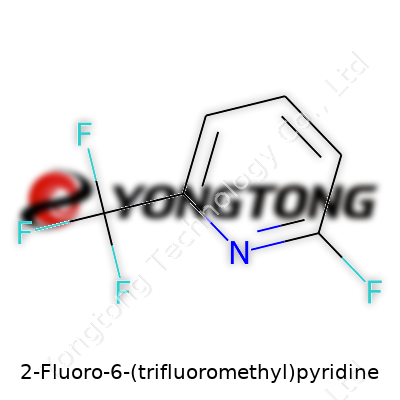
2-Fluoro-6-(trifluoromethyl)pyridine: Deeper Into a Modern Chemical Workhorse
Historical Development
The journey of 2-Fluoro-6-(trifluoromethyl)pyridine reflects the broader advances in organofluorine chemistry, catching the tailwind of post-war synthetic innovation. During the mid-20th century, breakthroughs in selective halogenation and metal-catalyzed reactions made it possible to introduce fluorine and trifluoromethyl groups into aromatic rings. As chemists searched for more stable, bioactive ingredients for agrochemicals and pharmaceuticals, interest in such molecules took off. Pyridine derivatives like this compound transitioned from obscure reagents to central intermediates, piggybacking on the expanded toolkits of the fluorination revolution.
Product Overview
2-Fluoro-6-(trifluoromethyl)pyridine, sometimes abbreviated as 2F6TFMP, belongs to a class of halogenated heterocycles. As someone who has spent years watching chemical trends, this molecule stands out for its rare combination of electron-withdrawing power and aromatic stability. You notice it surface as a multipurpose building block, lending versatility to labs looking for new pharmacophores or crop protection candidates. It doesn’t command the same spotlight as blockbuster drugs, yet it shapes the backbone of a whole suite of downstream molecules.
Physical & Chemical Properties
This compound typically appears as a clear, colorless liquid, sometimes giving off a faint, sweet, ether-like aroma that gives away its potent volatility. With a molecular formula of C6H2F4N, it sports a molecular weight close to 181 g/mol. Because of the electron-deficient pyridine ring and the strong pulling influence of both fluorine and trifluoromethyl groups, it shows low basicity compared to its simpler pyridine cousins. Its boiling point hovers around 120–130°C, and it isn’t very soluble in water, but mixes well with organic solvents like acetonitrile and dichloromethane. Hard data shows its stability under mildly acidic and neutral conditions, but trace alkali can provoke decomposition or undesirable nucleophilic substitution reactions.
Technical Specifications & Labeling
You’ll find this material usually offered at purities above 98%, tightly controlled through GC and NMR analysis. Reputable suppliers include detailed batch-specific CoA reports, often listing chloride, sulfate, and heavy metal residues well below 10 ppm. Proper UN shipping classification matters, given its combustibility and low flash point. Standard packaging involves amber glass bottles with airtight caps, frequently labeled with signal words like “Warning” due to potential health effects. My own experience dealing with industrial orders highlights the importance of dual labeling—common name and recognized identifiers like CAS number. The trend moves towards barcoding and RFID for improved inventory traceability and to support emergency response logistics.
Preparation Method
The mainstream route to 2-Fluoro-6-(trifluoromethyl)pyridine starts with fluorination of trifluoromethylated pyridine precursors. Often, chemists rely on Selectfluor or other electrophilic fluorination agents to achieve selective mono-substitution at the 2-position. Anhydrous conditions and strategic use of solvent mixtures minimize side reactions. Another pathway leverages copper-catalyzed cross-coupling, reacting 2-halo-6-(trifluoromethyl)pyridines with fluoride donors. It’s vital to purge oxygen, as radical chain reactions have a habit of introducing impurities or lowering yield. In laboratory practice, column chromatography and distillation take care of purification. In the scale-up context, fractional distillation or crystallization under controlled temperatures help teams isolate product with consistency.
Chemical Reactions & Modifications
The electron-withdrawing character of both the fluoro and trifluoromethyl substituents lessens the ring’s susceptibility to nucleophilic aromatic substitution, except at high temperatures or in the presence of powerful nucleophiles. The 2-fluoro position allows for displacement by organolithium or Grignard reagents under carefully controlled conditions, offering entry points for custom modifications. Hydrogenation of the pyridine ring forms partially saturated analogs that researchers use to study structure-activity relationships in drug development. Sometimes, attempts to reduce or functionalize the trifluoromethyl group run up against the rugged stability of this moiety, yet recent advances using transition-metal catalysis have opened up pathways to introduce new functional groups while maintaining the integrity of the ring.
Synonyms & Product Names
Besides the full systematic name, this compound surfaces in literature under such synonyms as 2-Fluoro-6-(trifluoromethyl)pyridine, 2F6TFP, and sometimes as 6-(Trifluoromethyl)-2-fluoropyridine. Regulatory and supplier listings often reference its CAS number, which forms the backbone for inventory and compliance management. Older catalogs might still use abbreviations that reflect legacy naming systems, so researchers should cross-reference authoritative sources to avoid mix-ups in ordering or documentation.
Safety & Operational Standards
Handling this compound demands respect for its moderate toxicity and volatility. Inhalation can irritate respiratory mucosa, and direct skin exposure weakens the lipid barrier, sometimes causing localized reactions. Splash-resistant goggles and chemical-resistant gloves offer reliable personal protection. Fume hoods equipped with HEPA and carbon filtration provide the safest environment for manipulations. Flammable liquid storage cabinets and tight-sealing waste containers keep risks contained in busy research sites. Safety Data Sheets describe the routes of exposure and recommend calcium gluconate gel for accidental contact. My years around small- and medium-scale chemistry labs have shown that proper air monitoring and weekly PPE checks cut down on exposure events.
Application Area
Pharmaceutical companies leverage this building block to construct new central nervous system active agents and antiviral backbones, exploiting the unique lipophilicity and metabolic stability conferred by the trifluoromethyl group. Agrochemical researchers use it to synthesize herbicide and fungicide candidates that challenge resistant strains of weeds and fungi. Chemical process engineers appreciate its reactivity profile, allowing varied modifications to suit complex synthetic schemes. Medicinal chemists prize its ability to tune solubility and membrane permeability, which translates into better oral bioavailability in drug candidates. Some companies develop advanced coatings and specialty polymers, benefiting from the improved chemical resistance and thermal stability found in derivatives built on this motif.
Research & Development
A decade ago, access to 2-Fluoro-6-(trifluoromethyl)pyridine depended on high-cost, low-yield routes. Thanks to investments in green chemistry and continuous flow synthesis, cost per unit has dropped, and more labs include it in their library screens. Current R&D pursues further improvements: catalytic cycles with less hazardous waste, simpler fluoride donors, and new asymmetric routes that support the buildup of enantioenriched scaffolds. Advanced computational models predict how alterations in substituent placement influence biological activity, so researchers use this backbone as a platform for rapid SAR (structure-activity relationship) studies. Experience on multidisciplinary projects convinced me that cross-talk between synthetic, analytical, and biological teams accelerates both hit identification and process improvement.
Toxicity Research
Early work on related pyridines flagged concerns about acute toxicity through inhalation and skin contact. Recent animal studies show LD50 values above those of more reactive halo-pyridines, yet chronic exposure raises questions about cumulative organ effects. Long-term inhalation studies in rodents point to mild hepatic stress at high doses. Allergenicity testing still sits at an early stage, and environmental persistence—driven by the trifluoromethyl group’s extreme stability—deserves continued scrutiny. Modern laboratories track airborne levels using automated sensors, and well-established glassware cleaning protocols minimize residual contamination risk in sensitive toxicity assays.
Future Prospects
Broader adoption in pharmaceutical and agrochemical pipelines depends on safer, more efficient synthesis and improved understanding of metabolic fate. Start-ups and university groups push boundaries in site-selective fluorination and late-stage functionalization, opening doors for custom-tailored derivatives with specific biological targets. Environmental scientists now study the breakdown products of highly fluorinated aromatics, aiming to design analogs that degrade faster without compromising function. Looking forward, machine learning and automated synthesis platforms promise even faster exploration of new chemical space based on the 2-Fluoro-6-(trifluoromethyl)pyridine core. As synthetic challenges and safety concerns evolve, industry and academia will need stronger collaboration to balance innovation, risk, and regulation surrounding this quietly powerful molecule.
The Skeleton of an Everyday Chemical
2-Fluoro-6-(trifluoromethyl)pyridine sounds complicated, but it starts with a simple backbone—a pyridine ring. This ring holds together six carbon atoms, five bonded in a loop, and a single nitrogen atom wedged in the circle, representing one of the foundation blocks of organic chemistry. Chemists everywhere lean into its structure for a reason. Pyridine shows up in vitamins, medicines, herbicides, and even the defensive chemicals some insects produce.
Modifying the Blueprint: Fluorine and Trifluoromethyl
Now, picture this ring. Someone armed with careful hands snaps a fluorine atom onto carbon number two. Not just that, a thick, stubborn trifluoromethyl group—three fluorines clinging to a single carbon—anchors to the sixth position. That’s the structure of 2-Fluoro-6-(trifluoromethyl)pyridine: a straightforward, stable framework with tiny changes that send shockwaves through how it behaves.
The power comes from small tweaks like these. Fluorine atoms put a charge of electricity into the molecule, shifting the way electrons dance across the ring. The trifluoromethyl group, heavy with even more electronegativity, tugs at the chemistry, sometimes making the entire molecule less reactive, sometimes channeling reactions with intense focus. In my own lab experience, even one of these changes can make a sleepy molecule jump into new chemical games.
Why Change the Structure?
Skeptics might ask—why load up a simple ring with all these fluorines? Biology gives the answer. Human bodies, fungi, bacteria—they all react differently to molecules with small tweaks like these. 2-Fluoro-6-(trifluoromethyl)pyridine provides drug designers, pesticide makers, and materials scientists a chance to fine-tune potency and selectivity. Data backs this up: over half of the world’s blockbuster drugs contain fluorinated rings like this one. That’s not a coincidence. Fluorine increases metabolic stability, helping drugs last longer in the body, reducing breakdown and often unwanted side effects. In agriculture, shifting the ring structure this way sometimes makes for powerful weedkillers that spare food crops.
The Safety and Environmental Balance
Not every change brings only benefit. Heavily fluorinated chemicals have raised eyebrows for sticking around in the environment. Once released, their chemical bonds often resist breakdown, collecting in soil and water. Industry reports and university studies both point to traces of these molecules showing up far from where people want them, including water supplies. The world now looks harder at the overall impact—me, I think about the gloves and ventilation fans I use in lab, but the conversation stretches farther, out to the dinner tables and riversides across the globe.
Building for Tomorrow
Solutions come from science that keeps pushing, rather than ignoring the risks. Chemists have a bag stuffed with tricks: biodegradable alternatives, new catalysts that limit waste, and careful rules about disposal. I’ve seen research teams swapping out stubborn functional groups for ones that break down after their job is done. Regulators can help by sharing clear data and setting realistic safety limits, not just for the makers but for everyone who ends up living next to a field or a stream downstream from a plant.
The main lesson? Precious little changes like a single fluorine or a clustered trifluoromethyl group don’t just transform a molecule—they touch the worlds of medicine, food, and the spaces we call home. That’s worth paying attention to, whether mixing chemicals in a beaker or reading the fine print on a bottle in the pharmacy.
Finding Value in Chemical Discovery
Working as a research assistant in an industrial lab taught me the difference a single molecule can make for an entire supply chain. Through one project after another, I saw how a tweak in structure brings new properties and possibilities. 2-Fluoro-6-(trifluoromethyl)pyridine stands out as a perfect example. This small ring transforms medicine, materials, and crop science beyond expectations.
Building Better Drugs
Drug discovery keeps facing tough demands—longer shelf lives, stronger activity, fewer side effects. Medicinal chemists reach for building blocks that change the game in subtle ways. This fluorinated pyridine brings both fluorine’s electron-withdrawing punch and a trifluoromethyl twist, nudging molecules toward higher potency or better selectivity.
It ends up as a core scaffold in antiviral, anticancer, and central nervous system agent candidates. Research papers from 2020-2023 cite this compound as a stepping stone for pyridine-containing kinase inhibitors and tuberculosis drugs. Its unique configuration helps boost membrane permeability, so drugs cross into cells more easily. That can mean lower doses, cheaper manufacturing, and less toxic off-target effects.
Crop Protection Solutions
Farmers look for tools that protect crops but don’t destroy the earth beneath their boots. A few years back, a colleague showed me patent applications using this molecule in the synthesis of herbicides that hit tough weeds without lingering in the soil. The trifluoromethyl group resists degradation, ensuring the active ingredient works exactly when and where it’s needed—not long after.
Companies use this pyridine variant as a key intermediate, especially in selective herbicides that target broadleaf weeds but spare grains. Its structure makes downstream chemical reactions more efficient, which keeps costs in check during large-scale production. In an industry racing against resistance, a new pyridine source keeps options open.
Materials Chemistry Means Progress
Materials science turns on new monomers and crosslinkers. Engineers and chemists working on new polymers and liquid crystals pay close attention to heterocyclic scaffolds like this one. The ability to fine-tune physical properties stands out here. If you want a chemical-resistant polymer film or an OLED display that lasts, this fluoro-trifluoromethyl building block helps.
I remember a case involving OLED emitters built from related fluorinated pyridines. The molecules raised device efficiency while lengthening lifespan, which mattered for phone and TV displays exposed to daily wear. Adding this particular motif serves as a launching pad for similar innovations in electronics, adhesives, and coatings.
The Path Forward
Innovation relies not only on what chemists can dream up, but also on what raw materials make available. 2-Fluoro-6-(trifluoromethyl)pyridine won’t fill news headlines, but its impact spreads across multiple sectors. Access to reliable sourcing, safe handling, and in-depth toxicology results could speed up its adoption in even more downstream products. For every lab hunting the next breakthrough, having trusted building blocks like this one makes all the difference between progress and dead ends.
The Value of Purity
Stepping into any lab or quality-focused facility, one clear expectation comes up: what’s inside the container matches what’s written on the label. Purity isn’t a buzzword—it's the measure of how much of a substance is the ingredient you are actually paying for. In pharmaceuticals, high purity keeps patients safe. In food production, purity stands between clean nutrition and unwanted contamination. Even in manufacturing, impurities can send entire product lines out of spec and trigger recalls.
Let’s put this into perspective. In the US, pharmaceutical standards demand 99% purity or higher. This strict level ensures that medication works the way doctors expect and limits side effects from unknown leftovers. Chemistry labs handling reagents often require similar purity—trace elements, down at the parts-per-million level, can throw off an experiment or make test results unreliable. Impurities in food ingredients sometimes pose allergy risks or lead to spoilage.
Numbers on certificates of analysis should always match the batch that arrives. Sometimes I’ve found bulk chemicals labeled “99%” from one supplier perform better or worse than another, just due to differences in trace contaminants. Basic due diligence—reading third-party test results and knowing where ingredients come from—gives anyone purchasing raw materials some peace of mind.
Physical Form: Practical Impact
Another side of the story rests in the material’s physical form. Granulated sugar and powdered sugar, sodium chloride in pellets versus crystals, or active ingredients delivered as tablets or powders—each fits a different job. For someone in food processing, granulation changes everything from mixing time to how flavors blend. In my work, I’ve watched how the shape and size of solid ingredients influence how easily they dissolve in solutions or disperse for coatings.
Pharmaceuticals need exact particle sizes—too coarse, and tablets crumble; too fine, and powders might become airborne. Liquid formulations have their own issues: particles settling, separating, or reacting if storage conditions shift. I once saw a production line grind to a halt because the form factor wasn’t right—a supplier sent crystalline material where powder would’ve blended easily. Time and money lost, all because no one double-checked the packing slip.
Physical form matters in storage, too. Granules flow differently from flakes or large crystals, affecting automation in production facilities. Some forms absorb moisture faster, increasing spoilage or clumping. Products engineered for pharmaceutical injection depend on the solubility of the source compound, which links straight back to particle size. One overlooked detail can ripple through an entire process.
Getting It Right
To avoid costly surprises, it helps to communicate up front what specifications are non-negotiable. Clear documentation and regular checks with suppliers go a long way. I’ve heard of teams working with two suppliers at once, then measuring batches before every production run. If a batch varies, they call it out and request a replacement—no middle ground. Keeping samples for reference and comparing new deliveries with past lots tightens control.
Bringing in expert guidance matters, especially for new labs or small businesses. Sites like FDA.gov and the European Pharmacopoeia list current standards. Networking with peers adds another layer of confidence—I picked up more than a few insights from colleagues sharing real-world stories, not just what’s printed on certificates.
Establishing a regular testing routine, trusting suppliers with a proven track record, and putting quality at the top of priority lists all help build safer products, minimize waste, and let teams focus on moving forward rather than cleaning up mistakes.
Why Storage Matters for This Chemical
Work in chemistry labs brings plenty of responsibility. The safety of people and the quality of research ride on good storage habits. 2-Fluoro-6-(trifluoromethyl)pyridine calls for attention, since it comes with unique risks like flammability and the potential for toxic exposure. Having spent years in research settings, I learned not to take shortcuts with safety. Colleagues have seen accidents happen when team members became too casual with chemicals.
This compound, with a fluorinated aromatic structure, often finds use in pharmaceuticals and specialty synthesis. While stable, it reacts poorly with moisture and strong bases. The dangers of ignoring proper storage reach beyond ruined product—a single mishandled vial can threaten everyone nearby. Following best practices reduces that risk.
Choosing Containers and Placement
Glass containers with airtight, tightly-sealed screw caps make the best home for sensitive chemicals. Fluorinated compounds like this one are notorious for corroding lower-grade plastics. Even minor leaks can cause fumes to escape, making proper sealing essential. Labels should be waterproof and include both the full name and hazard information, not just a chemical formula.
Temperature control plays a big role. Room temperature usually suits this compound, but extremes invite trouble. High temperatures can lead to slow decomposition and pressure buildup, while cold environments risk making vials brittle—especially with some glass types. I always kept such solvents and intermediates in a chemical storage cabinet away from sources of heat, far from direct sunlight. Humidity can sneak in and ruin sensitive compounds; a desiccator helps keep things dry. In damp climates, a lab fridge (never a food refrigerator) often becomes the storage spot of choice. Routine checks helped me spot condensation before it turned into a bigger mess.
Avoiding Unwanted Reactions
Isolation is not just a rule for highly reactive agents. Mixing storage for acids, bases, oxidizers, and organics leads to cross-contamination and hazardous mistakes. Dedicated flammable storage cabinets with key locks keep surprises to a minimum. I remember watching a close call unfold in a crowded stockroom when incompatible bottles were shelved together; fumes swept through, and it took days to clear out. Keeping 2-Fluoro-6-(trifluoromethyl)pyridine away from strong bases and acids works best, and double containment in trays adds another layer of security. Spills do happen, and a tray will catch most accidental leaks before they go anywhere dangerous.
Mindful Handling Lowers Risks
Proper protective equipment saves more than just skin. Nitrile gloves, lab coats, and splash-proof goggles offer protection. I favored working with such substances inside a fume hood—ventilation carries away any vapors. That practice lowers the chance of inhalation or surprise splashes. Keeping small quantities close at hand, but not overstocking, means less chemical to worry about if a shelf slips or a box accidently drops.
Building Better Habits in the Lab
New and experienced staff both need refreshers on chemical hygiene. Posting storage guidelines near supply cabinets reinforces caution. Clear logs tracking who takes and returns each bottle foster accountability. By keeping teams involved in regular safety audits, labs minimize risks and create a culture where everyone looks out for each other.
Responsible storage of 2-Fluoro-6-(trifluoromethyl)pyridine upholds both safety and scientific integrity. Paying attention to container quality, clear labeling, climate, and handling habits reduces danger and waste, making chemists’ lives a whole lot safer in the long run.
The Role of CAS Numbers in Science and Everyday Business
People throw around chemical names and formulas, but only a handful outside chemistry labs pay attention to the numbers that travel along with them—those nine-digit CAS numbers. Take 2-Fluoro-6-(trifluoromethyl)pyridine. Its CAS number is 198436-45-2. This isn’t just a set of digits. For researchers, manufacturers, and regulators, this number zips straight to the exact compound, skipping the muddle of synonyms and misspellings.
With so many chemicals masquerading under different names, sorting them efficiently seems impossible without a tool like the CAS number. Think about how names in chemistry grow wild—“2-Fluoro-6-(trifluoromethyl)pyridine” might be written a few different ways depending on the journal, supplier, or country. A unique code slows down confusion, strips away ambiguity. This is particularly valuable for global chemical supply. A U.S. lab may order a sample, while a colleague in Japan requests more of the same, and the shipment will match—because everybody speaks the same “language" in this case.
Trust and Traceability: Foundations of E-E-A-T in Chemistry
Every person who’s had to look for a rare compound feels the frustration of unreliable sources and old stock lists. The community values accuracy and trust, especially when health, safety, and reproducibility are on the line. The CAS registry sits with over 270 million substances. The moment a chemist uses the code 198436-45-2, the rest of the world knows exactly what’s in that flask, or, if they don’t, they can look it up at once through multiple reputable databases. This creates a chain you can verify, so mistakes that could lead to wasted funding or, worse, dangerous accidents, become less likely.
Experience in a small research group taught me that nothing throws a wrench in a project quite like the wrong chemical—especially with tricky fluorinated pyridines. Once, a colleague ordered a compound with nearly the same name as what we needed. Close wasn’t good enough. Experiments failed, weeks disappeared, and everyone felt the stress. Since then, CAS numbers have become non-negotiable in every order, record, and publication.
Problems with Access and Data Integrity
Almost every field struggles with information overload and outdated or inconsistent records. Chemistry faces a similar headache. Free databases sometimes lag, and companies across the globe list the same compound with minor spelling errors, leading to incorrect deliveries or regulatory drama—especially in pharma, agrochemicals, and electronics. Human error adds another layer of risk.
Solutions start with better training and full digital integration. Reliable suppliers match chemical listings directly with the CAS number at every point in the supply chain. Laboratories and procurement offices embed those numbers in inventory tags, recipes, and reports. Open access and clear guidelines from regulatory bodies, along with wider adoption of validated digital records, keep mistakes from multiplying.
Bottom Line for Industry and Research
Keeping track of chemicals using unambiguous IDs matters even more as research speeds up and regulations tighten. The CAS number for 2-Fluoro-6-(trifluoromethyl)pyridine, 198436-45-2, locks in certainty, builds trust, and saves time. Chemical safety, accurate communication, and rapid progress all depend on it.
The Hunt for High-Quality Chemical Compounds
Anyone who's spent time in a chemistry lab knows that purity is not just a technical term. It's the difference between repeatable results and frustrating misses. 2-Fluoro-6-(trifluoromethyl)pyridine draws attention from research groups and industrial manufacturers, especially those working with pharmaceuticals and specialty plastics, because that little tweak—purity—tells you whether your synthesis keeps on track or veers off into a tangle of unknowns.
From practical experience, chemists rarely take what a label claims at face value. Many chemical suppliers list 98% as the default purity for compounds like this pyridine derivative, and calling that number good enough has become common in the industry. Some specialty vendors promise 99% or higher, especially if drug or electronics work demands higher standards. I always tell people: Don’t just stare at the catalog number—check the supplied certificate of analysis (COA). That snapshot of quality, shaped by GC, HPLC, or NMR data, tells you much more than a two-decimal number ever could.
Tracing Contamination Sources
Small impurities sometimes take up far more attention than their mass would warrant. Even a quarter-percent contaminant can play havoc in drug discovery or OLED manufacturing. Many compounds ride in on the backs of synthesis remnants or storage breakdown. In practice, a lab can receive material that’s “98% pure,” but sensitive tests like NMR can reveal trace organics or fluoride leaks lingering from the synthetic path. The labs that struggle least usually ask for updated analytical data before big orders.
Why Purity Sets the Tone for Research
Most scientists find that going cheap on purity early on invites expensive troubleshooting later. A lower-purity compound may function as a model molecule for academic study where high throughput isn’t critical. The moment someone shifts their work toward active pharmaceutical ingredients or high-performance materials, the purity bar rises. Impurities can mimic reactants, clog up columns, or spoil delicate catalytic cycles. Pharmaceutical regulations punish a lack of rigor, driving a whole industry of contract labs whose job is to verify—again and again—that the white powder in one bottle matches company safety and performance promises.
Ways Laboratories Can Raise Confidence
Back in grad school, I learned the hard way that buying high-purity material gives you an edge, but verification is king. For 2-Fluoro-6-(trifluoromethyl)pyridine, I recommend asking for detailed COAs and pushing for NMR spectra or chromatograms with every batch. If you’re running something critical, order a small amount, analyze it yourself, then scale the order. Some major suppliers even support pre-shipment HPLC reports if you ask. Yes, this means more upfront work, but it saves headaches later—a lesson worth repeating for any research group or manufacturer with tight budgets or strict quality standards.
Toward Shared Standards and Better Transparency
No lab wants to toss out a month of work over a hidden contaminant. The move toward open data on chemical sourcing, and even third-party verification, stands to give researchers more certainty. Suppliers listening to industry feedback now provide more detailed purity breakdowns and reveal breakdown products in technical documentation—a step that can prevent ruined synthesis and ensure everyone marches to the same quality drumbeat.
Understanding the Compound
2-Fluoro-6-(trifluoromethyl)pyridine doesn’t look dangerous by name alone, but that fluorine-loaded structure packs a punch in the lab. It’s used in all sorts of chemical syntheses and pharmaceutical projects. If you've ever walked into a chemical storeroom, you’ll recognize the distinct, crisp attitude of anything trifluoromethyl—volatile, reactive, and not to be left out in the open.
What Does Proper Storage Mean?
I remember the days when glass bottles lined the shelves and labels started to peel. That kind of sloppiness leads to nasty spills or degraded materials. For 2-Fluoro-6-(trifluoromethyl)pyridine, storage isn’t just about cleanliness—it’s about protecting researchers, preserving integrity, and avoiding environmental headaches.
Room temperature in a cool, dry place usually works. That means between 15–25°C, away from heat sources and direct sunlight. Humidity doesn’t play well with this compound, so basements or steamy setups turn risky fast. Desiccators or low-moisture cabinets prove themselves every time.
Anyone who’s ever opened a leaky bottle knows why tight caps and solid seals matter. This compound gives off fumes that never belong in open air. If you catch a whiff, ventilation hasn’t kept up. Chemical fume hoods save more headaches (and lungs) than most folks realize, especially if any transfer happens outside original containers.
Why Segregation Matters
Mixing incompatible chemicals can end badly. Pyridine derivatives might not explode with a gentle breeze, but placing them next to acids, oxidizers, or bases is a rookie move. Flammable cabinets lock hazardous materials away from sparks and casual contact—keeping 2-Fluoro-6-(trifluoromethyl)pyridine in its own spot, away from nitric acid or bleach, lets you avoid dangerous reactions.
If you have to store lots of substances, colored or coded labels go a long way to stop mistakes. Inventory records should stay updated—there’s nothing more frustrating than running out or using spoiled material for a critical project. Regular checks mean researchers catch crystallization, color changes, or pressure build-up before anything serious kicks off.
Handling and Environmental Responsibility
Spills and leaks cause bigger issues than most realize, especially with fluorinated chemicals. In my experience, even small drops on the bench linger in the air and on surfaces unless cleaned up with the right gear. Absorbents and spill kits should stay within arm’s reach, and everyone should know how to use them.
Some labs set policy on secondary containment, like trays beneath bottles, to catch leaks. This takes only a few extra seconds but saves hours of decontamination. Large volumes demand special attention—buried deep in regulations sits the reality that local environmental protection folks take a dim view of improper storage.
Safer Practices = Better Results
Good storage habits mean less waste, more reliable results, and safer employees. Clear instructions, frequent training, and a bit of healthy skepticism all serve the same goal—keeping both people and projects on track. When chemicals like 2-Fluoro-6-(trifluoromethyl)pyridine are handled with respect, accidents happen less often and work gets done right.
All these small steps—right temperature, sealed containers, good ventilation, smart segregation, spill readiness—aren’t just box-ticking. They beat back chaos and make sure everyone gets home in one piece. For labs looking to build trust, earn credentials, or just finish a synthesis without disaster, nothing substitutes for good storage discipline.
Why Bulk Chemicals Matter in R&D and Industry
Sourcing specialty chemicals often feels harder than developing what you want to make in the lab. 2-Fluoro-6-(trifluoromethyl)pyridine, a mouthful of a name, counts as one of those compounds that’s always somewhere on a wish list for pharma, agro, and advanced materials projects. I’ve seen chemists spend days scouring supplier catalogs or negotiating tiny samples from abroad, often at eye-watering prices. Companies that rely on complex synthons like this one require steady supplies or risk breaking their development timelines.
Availability and What Drives Access
Sitting in a purchasing role years ago, I realized something: many distributors only keep enough stock to meet niche demand. 2-Fluoro-6-(trifluoromethyl)pyridine falls into this gray area—not a true commodity like acetone, not exotic enough to spark custom manufacturing every month. Large chemical marketplaces like Sigma-Aldrich, TCI, and Alfa Aesar provide this compound in gram-to-multi-hundred-gram packs. Sometimes bulk means just a few hundred grams; bigger orders trigger a long lead time or require special quotes. That’s a speed bump for any group trying to scale from proof-of-concept to pilot plant synthesis.
On the producer side, a handful of Chinese, Indian, and European firms offer bulk quantities by the kilo, sometimes with minimum order sizes starting at ten or twenty kilos. Quotes jump dramatically compared to lab-scale pricing, mainly because these suppliers either make small batches per order or ship direct from plant inventory, not a warehouse shelf in the customer’s country. In my own experience, orders above five kilograms often bring up questions about purity, packaging, and transport regulations—hazardous goods rules really ramp up shipping costs and slow customs clearing.
The Reliability Factor
It’s one thing to find product listings online—it’s another to get what you paid for. Over the years, I’ve learned the hard way to not blindly trust every catalog. Authenticity checks, certificates of analysis, sample test runs, and reference spectra with reputable labs turn up sometimes glaring differences in product quality, even for the same compound. For 2-Fluoro-6-(trifluoromethyl)pyridine, the trickiest part can be tracing impurities like remnant solvents or side-products from specific fluorination routes. Consistency matters, especially in pharma and electronic applications, where an unseen impurity can derail synthesis or distort material properties.
Handling the Sourcing Challenge
Labs and companies short on in-house procurement expertise need to spend time vetting manufacturers, not just comparing prices. Trusted distributors give faster access but charge a premium. Direct-from-plant bulk brings cost advantages for high-volume users, but payment terms, export experience, and documentation quality can vary wildly by region. Third-party testing, on-site audits, and clear contracts make a big difference. I always recommend close communication upfront about batch tracking, re-order timelines, and shipping arrangements before moving beyond sample stages.
Online sourcing platforms have reshaped the process, offering direct search and quote features, but the basics hold: strong relationships, technical transparency, and readiness to pay for quality trump chasing the lowest price. For 2-Fluoro-6-(trifluoromethyl)pyridine, a little extra effort in qualification checks usually pays off in fewer delays, fewer failed reactions, and more predictable costs on scale-up. In an industry where one bottlenecked reagent can stall a whole pipeline, reliable bulk supply becomes less a luxury and more a necessity.
Why the CAS Number Matters in Chemical Work
Everyone who’s spent time managing chemicals, whether in a research lab, a pharmaceutical plant, or a startup working on green tech, comes face-to-face with the need for clarity. You run into a family of chemicals, and each one looks pretty similar at a glance. Take 2-Fluoro-6-(trifluoromethyl)pyridine. It’s a mouthful, and the name hints at some tricky rings and tricky fluorinated bits. But even with a detailed chemical name or structure, there’s always wiggle room for confusion. That’s where the CAS number, a unique identifier, earns its keep. This trackable, fixed label cuts out the guessing better than any name ever could. For this compound, the CAS number is 22250-20-0.
How This Identifier Shows Up in Real Life
I remember sorting through catalogs in a previous job, hunting for chemicals. Each vendor called things differently—sometimes the same compound popped up under three listings, all spelled a little differently, or even with a missing dash. Quality control relied on getting that CAS number right, not just the name or structure. Once that number got plugged in, every database aligned, every bottle on the shelf matched up, and accident risk dropped. In a field where even a tiny switch in the placement of a fluorine atom or a methyl group means big changes in toxicity, reactivity, or cost, this level of precision keeps people safe.
Trust in Science Relies on Traceability
Think about the ripple effects. Journals demand reproducibility; safety officers expect full documentation; regulators look for compliance. In any of these circles, chemicals without clear tracking create stumbling blocks. The CAS number becomes the checkpoint—no confusion, no doubt. When colleagues ask for 2-Fluoro-6-(trifluoromethyl)pyridine, offering the number 22250-20-0 makes sure the right compound arrives. This isn’t just bureaucracy. One mistake on a label, one mix-up during ordering, could mean hours of wasted work or worse—serious hazards or regulatory trouble.
Discussions About Chemical Sourcing
Markets for specialized chemicals sometimes feel scattered, especially for less mainstream compounds like highly fluorinated pyridines. Suppliers in different countries sometimes label things according to local conventions. Search engines or procurement systems don’t always interpret the complex IUPAC nomenclature the same way. So folks in the know stick to CAS numbers, trading them across emails, database fields, and regulatory forms. Reliable sourcing depends on people finding the exact thing they want, whether for synthesis, development, or testing. That accuracy also speeds up customs checks, storage labeling, and disposal decisions.
Keeping Workflows Safe and Smart
Accidents or compliance incidents suck up resources and shake confidence in your workflow. Getting the CAS number right from the start chases away a whole category of error. Digitizing chemical inventory adds another layer—scanners, mobile apps, and regulatory portals all pull up suppliers and safety data instantly, based on this identifier. As supply chains become more global and traceability rules get tighter, the humble CAS number keeps the gears running. There’s a reason no one in the business ignores it.
Final Thoughts: A Foundation of Trust
Every day I see new grads or researchers shrug at numbers that look like barcodes. Truth is, each one carries a guarantee behind it. For 2-Fluoro-6-(trifluoromethyl)pyridine, knowing its CAS number—22250-20-0—turns a complicated name into a concrete entry in any reputable system. It saves time, forestalls mistakes, and keeps compliance simple. Small detail, big payoff.
Understanding the Risks
People in research labs tend to see bottles of 2-Fluoro-6-(trifluoromethyl)pyridine as just another routine fluorinated reagent. It's easy to treat familiar chemicals casually; after a dozen syntheses or late-night runs, the steps can feel almost automatic. Experience tells a different story, though. This compound blends the volatility of fluoro-organics with persistent toxic effects. Even a little spill stinks, stings, and lingers. It's not just about personal risk; contamination spreads quickly, and these residues stick around for weeks if missed during cleanup.
Why Proper Handling Practices Save Trouble
The material comes as a colorless liquid, low viscosity, and a tendency to evaporate fast. Without proper ventilation, exposure builds up quietly. Fluorinated aromatics cause respiratory problems, skin irritation, and eye burns. I once worked with someone who ignored a split-seal on the fume hood sash. They opened a bottle near the bench, thinking a couple of breaths wouldn't matter. They coughed, their throat burning, and we paused everything until the air cleared. Accidents like this are avoidable with clear routines.
Engineering controls lead the pack: chemical fume hoods with checked airflow, glove boxes for large-scale handling, and sealed waste containers. Nitrile gloves, splash-free goggles, and labcoats stand as the bare minimum. The liquid migrates into nitrile eventually; regular glove changes keep skin safe. I’ve seen regular latex fail spectacularly with these compounds, so cutting corners never pays.
What the Data Says
The manufacturer’s safety sheets tell plenty: low flash point, moderate vapor pressure, and substantial systemic toxicity. Inhalation causes dizziness, nausea, and can even knock a person unconscious if concentrations spike. Direct skin exposure triggers inflammation or chemical burns. Most labs keep calcium chloride or other neutralizing spill agents on hand because water alone does not always work for fluorinated pyridines. Flammable liquids cabinet storage reduces risk during earthquakes or fires; all containers need good seals and labels that don’t vanish after a minor ethanol splash.
Thinking Beyond the Procedures
Training new staff goes a long way. It’s easy to become complacent, so running a spill drill twice a year keeps everyone sharp. Clear signage over storage shelves helps people double-check containers. Eye-wash stations and emergency showers can’t just collect dust in the corner, not with organofluorines on the shelves. Even as labs get busier and deadlines pile up, everyone walking in knows the value of a shared safety culture.
Waste disposal often gets skipped in the rush to finish an experiment. Fluoro-organic solvent waste takes extra care—secondary containment, accurate labeling, and sealed drums make the difference between a safe disposal process and a real headache when the hazardous waste crew does their monthly tally.
Building a Culture of Respect
Years spent around synthetic benches teach a healthy respect for reagents like 2-Fluoro-6-(trifluoromethyl)pyridine. Slowing down, reading the label, double-checking the hood—these steps keep your lungs, skin, and labmates out of trouble. Investing a few extra seconds for safety now saves hours, sometimes days, of incident clean-up and health checks. Taking chemicals seriously doesn’t just protect one person; it shields the whole lab from months of hassle.