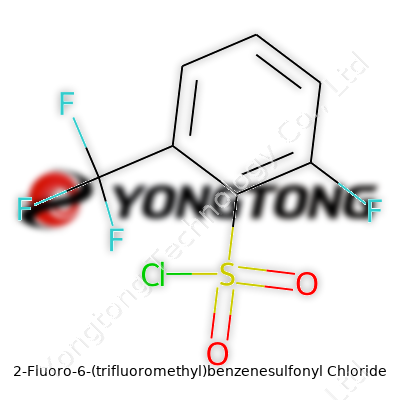
2-Fluoro-6-(trifluoromethyl)benzenesulfonyl Chloride: A Modern Chemical Powerhouse
Historical Development
Synthetic chemistry has always looked for new building blocks to push pharmaceutical and material science forward. Back in the 1970s and 1980s, fluorinated aromatics emerged as top candidates in labs due to their unique reactivity and stability. The path toward more specialized reagents sharpened, and by the late 90s, researchers had eyes on compounds like 2-fluoro-6-(trifluoromethyl)benzenesulfonyl chloride. Unlike older sulfonyl chlorides, this one brings together two different fluorinated substituents, both widely valued for boosting molecular performance. The introduction of a trifluoromethyl group and an ortho-fluorine atom didn’t just happen by accident. Chemists realized that stacking electron-withdrawing groups this way led to better leaving group characteristics, greater resistance to breakdown, and new opportunities for tuning reactivity. Early adopters pushed through tough, multi-step syntheses, and gradually, more efficient routes appeared. Today, this compound stands as a testament to a kind of problem-solving that values both function and creative process.
Product Overview
2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride isn’t another basic reagent sitting on a dusty shelf. It brings a trifecta: strong electrophilic power (thanks to the sulfonyl chloride), increased metabolic stability from the fluorines, and an ability to tune performance in crowded chemical spaces. Drug designers and agrochemical researchers often look at this molecule because it slots into synthesis plans where standard sulfonyl chlorides fall short. The trifluoromethyl group makes it more than a simple source of sulfonylation—it lets end products handle metabolic challenges that defeat old-fashioned analogs. In a world where fine differences can mean millions of dollars in drug development, this stuff earns its place.
Physical & Chemical Properties
From experience, working with 2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride almost feels like having a "super-reagent" around. It usually appears as a pale yellow liquid or sometimes a crystalline solid at room temperature, with a menacing, acrid odor that you never forget once you’ve met it. It weighs in at about 262 grams per mole, and with a boiling point above 140°C (at reduced pressure), you don’t have to rush your operations. The molecule’s three fluorines and that extra fluorine at the ortho position crank up its resistance to hydrolysis, so it doesn’t collapse if a little moisture sneaks in. I’ve found it stays workable in ambient conditions, although it still demands the kind of respect any sulfur-chlorine group deserves. Its high electronegativity, shaped by the adjacent fluorines, makes it far more reactive than basic sulfonyl chlorides.
Technical Specifications & Labeling
Suppliers don’t get away with lax labeling here, thanks to regulatory expectations. The compound usually arrives with purity above 98% by gas chromatography, and every bottle carries warnings about moisture, light, and air exposure. You’ll usually see batch specifications including melting point (if it’s solidified), water content (Karl Fischer titration), and data from NMR or mass spectrometry to confirm identity. Frankly, the tight quality control isn’t just red tape; it shields end-users from wasted time and unpredictable results, which can spiral costs in a research lab. Labeling also notes its incompatibility with strong nucleophiles outside reaction plans—nobody enjoys surprise exotherms in a busy lab.
Preparation Method
On the production side, chemists often start with 2-fluoro-6-(trifluoromethyl)benzenesulfonic acid or the corresponding aniline. Chlorination with reagents like thionyl chloride or phosphorus pentachloride turns the acid into a sulfonyl chloride. Yields reach up to 85% under dry, controlled conditions, though the choice of solvent and temperature can push numbers higher or lower. I’ve seen some manufacturers dodge hazardous reagents by using oxalyl chloride, which is a bit less problematic to handle in scale-up. It isn’t glamorous work, but careful attention to purification, with distillation under reduced pressure or flash column chromatography, makes the difference between a useable batch and a shelf full of mystery oil. Some recent innovations at scale even capture hydrogen chloride byproduct, feeding it into neutralization systems on-site.
Chemical Reactions & Modifications
This sulfonyl chloride steps into electrophilic aromatic substitution or sulfonamide formation like a pro. Anyone in organic synthesis quickly learns how keen it is to react with amines to form sulfonamides, a crucial scaffold in both pharma and materials chemistry. The presence of multiple fluorines dials up both selectivity and yields in coupling reactions, especially where steric hindrance gets in the way for regular aryl sulfonyl chlorides. I’ve watched chemists swap in this building block to create protease inhibitors, develop tough polymer backbones, and even build new rechargeable battery materials. Its chemical temperament means you can introduce other functionalities post-sulfonylation, such as Suzuki coupling, to attach new aromatic or heterocyclic groups. Some labs also exploit its unique UV-absorption properties for tagging and detection, tracking reaction progress with little extra equipment.
Synonyms & Product Names
Anyone shopping for this compound soon discovers it travels under multiple aliases across catalogs. Some call it 2-fluoro-6-(trifluoromethyl)benzenesulfonyl chloride, others use the more systematic name: 2-fluoro-6-(trifluoromethyl)benzenesulfonic acid chloride. In trade, a few suppliers shorten it to TFMSC or use product codes in their batches. Having tested quality from five or six vendors, I’ve learned to check not just the names but also the CAS numbers, since confusion still happens in global commerce where similar sulfonyl chlorides jostle for attention. This habit of cross-checking avoids expensive mix-ups in multi-step syntheses.
Safety & Operational Standards
Sulfonyl chlorides don’t forgive sloppy handling, and this one is no exception. It reacts with moisture to release hydrogen chloride—a nasty scenario for both operators and lab hardware. Gloves, splash goggles, and well-fitted lab coats are the daily armor. Proper fume hood discipline can’t be skipped; inhaling even a whiff spells trouble for nasal passages and lungs. Material safety data sheets highlight acute irritancy to skin and eyes, with chronic exposure risks flagged for respiratory tissues. In scale-up, exhaust scrubbing and emergency wash stations are essential. Spills mean swift neutralization with sodium bicarbonate or another suitable base, not just mopping up. From experience, skipping even one step in the safety protocol tends to come back to haunt you by the end of the day.
Application Area
Chemists in pharmaceuticals love this molecule for the elegant way it creates metabolically stable linkages in potential drug candidates. The world’s top-selling diabetes and anti-inflammatory agents often hide a sulfonamide somewhere deep in their structure, and many are derived from compounds like this. Material scientists see different advantages: the fluorinated aromatic groups transfer to polymers for improved chemical resistance, hydrophobicity, and even better charge transport in battery materials. Agricultural chemical developers benefit from the persistent, weather-stable nature that fluorine delivers here, rolling out new herbicides or growth regulators that don’t wash away so easily. In my own work, I’ve seen its use expand beyond the obvious, popping up in dyes and markers for biological assays, even as an intermediate in photovoltaic research. Across the board, it’s a tool for problems that outwit simpler molecules.
Research & Development
Recent years have pulled this compound into increasingly sophisticated projects. Medicinal chemists fine-tune its introduction to minimize off-target binding or metabolic breakdown, using it only where robust protection or unique reactivity pays off. Research into photoactive and electroactive polymers often relies on its presence to boost both strength and overall stability, while analytical chemists push new limits in detection and quantification, leveraging its UV and fluorine NMR signatures. I’ve participated in brainstorming sessions where its functional group diversity opened doors in protein engineering, putting it into protein inhibitors with a custom-fit design. Labs faced with persistent resistance and metabolic enzymes choose it to build molecules that last longer in biological systems without compromising safety. Each year, more papers show creative applications that didn’t exist a decade ago, from smart diagnostics to precision catalysts.
Toxicity Research
Toxicologists keep a close watch on all sulfonyl chlorides, especially ones carrying stubborn fluorinated groups. Test results found low acute toxicity in rodents when handled carefully, although eye and respiratory irritation comes up strongly in every report. Chronic testing sometimes reveals low-level organ impacts with repeated high-dose exposure, which is enough to keep bulk production well-regulated. Importantly, finished products that incorporate the sulfonamide often show much less toxicity—fluorination helps reduce off-target metabolism while also blocking certain breakdown pathways. From an environmental angle, breakdown products have to be tracked, since fluorinated aromatic fragments linger longer than simpler alternatives. Regulatory scientists dig into each new application, pushing for robust risk management in both lab and commercial settings.
Future Prospects
The spotlight on 2-fluoro-6-(trifluoromethyl)benzenesulfonyl chloride only keeps growing. Innovators know that site-selective fluorination is an arms race, both for newer therapeutics and more durable materials. Synthetic methods are getting faster, greener, and less dependent on legacy chlorinating reagents, adding extra appeal. Academic labs and startups alike broaden its reach with creative use in high-throughput screening, combinatorial libraries, and specialty coatings for extreme environments. It’s likely that biocatalysts or engineered enzymes will soon push boundaries even further, allowing for more controlled modifications and niche products. As demands for better medicines and safer materials grow, this compound stands ready for the next challenge—whether it’s in smart drug design or tomorrow’s not-yet-invented gadgets.
Understanding the Structure
Chemistry has always fascinated me, especially the way small tweaks in a molecule can turn the whole story around. Take 2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride, for example. Its name alone gives plenty to unpack, and every chemistry student sooner or later wrestles with such names that sound more like a mouthful than a molecule. Still, the beauty of IUPAC nomenclature reflects the molecule’s architecture.
The core here is a benzene ring—nothing fancy on its own—but now, imagine it dressed up with three distinct visitors: a fluorine atom at position two, a trifluoromethyl group at position six, and the gritty benzenesulfonyl chloride group. Each group offers a different flavor, both in chemistry class and in the lab.
Busting Down the Formula
Let’s piece it together. The benzene ring gives C6H6 as a starting point. Tack on a fluorine atom—C6H5F. Drop a hydrogen at position six for the trifluoromethyl group (CF3) and you’re looking at C6H4F + CF3—making it C7H4F4. Now, to attach the sulfonyl chloride group—a heavyweight in sulfonation chemistry, punching in with SO2Cl. That brings the totals to C7H4F4SO2Cl.
I once spent hours in the lab working on aromatic sulfonyl chlorides for a project. The key is treating the formula not as some random string, but as a map to the actual molecular world. When you look at 2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride, the formula C7H3ClF4O2S tells you exactly what's getting stirred in your reaction flask.
Why Structure Matters
This precise arrangement shapes the way chemists approach synthesis and reactions. The trifluoromethyl and fluoro groups deliver big changes. From working in pharma, I’ve seen how a single fluorine atom jammed into a benzene ring can completely change a drug’s behavior in the body. Add a trifluoromethyl group, and suddenly it’s more resistant to metabolic breakdown or slips through cell membranes with ease.
The sulfonyl chloride part pops up everywhere in synthetic organic chemistry—think of it as a heavy-duty handle that lets you tack on all sorts of useful fragments. It transforms simple aromatics into active pharmaceutical ingredients, engineering materials, agrochemicals—the list goes on.
Challenges and Paths Forward
Accurately writing a formula seems simple until you’re in a rush or distracted. I’ve watched seasoned chemists miss a fluorine or swap a position, which leads to confusion or wasted resources. Chemical databases rely on accuracy, so getting formulas correct supports research integrity. Double-checking every character feels tedious, but it protects against a chain of bad data that could ripple through experimentation and publication.
Teaching helps too. Many educators walk their students through examples like this so newcomers grasp why each part of the molecule matters, not just what the letters and numbers spell out. Smarter educational tools that visually link names and formulas help turn textbook frustration into deep understanding.
Societal Impact
Complex molecules like this drive progress in medicine and materials science. Getting them right on paper and in practice means better drugs, safer pesticides, and more advanced surfaces—contributions that ripple out to all corners of life. Precision in structure and formula isn’t just an academic quest; it’s the quiet engine that moves science forward.
Chemical Formula: C7H3ClF4O2SInside the Home and Community
If you check the pantry, there’s a good chance you’ll spot something containing this compound. It works as a stabilizer in prepackaged bread and sprinkled over some cheeses to keep them from clumping together. Some cleaning products rely on it to soften hard water, making laundry detergents a lot more effective. I remember living in a college dorm, washing my clothes with plain water and soap, and it just didn’t work half as well—hard water always leaves behind residue. This compound solves that by binding with minerals, so soap has a real chance to do its job.
Cities add this ingredient to drinking water for several reasons, including corrosion control in old pipes. Without it, old lead pipes might release harmful metals, so keeping tap water safe sometimes comes down to a few grams of this chemical mixed in at the water plant.
Medicine and Health
Drugmakers use this compound to keep pills from breaking apart inside the bottle. When my grandmother was prescribed heart medication, her pharmacist explained that the white powder coating kept the active ingredient dry and prevented clumping. Hospitals turn to this compound to treat certain overdoses and heavy metal poisoning; doctors prescribe it as a chelating agent that helps the body remove lead or mercury. The World Health Organization has recommended it for several decades, so its safety profile is pretty well documented.
Dentists sometimes talk about how this chemical keeps mouth rinses and some toothpastes stable. A little goes a long way, but it’s another example of boring chemistry quietly improving lives. Even baby formula makes use of it for mineral balance, following strict safety limits.
Industry and Manufacturing
Factories can’t do without this chemical, especially when making textiles or paper. During my summer breaks, I worked in a textile plant. The dye process called for consistent water quality, which wasn’t possible in our area’s hard water. By adding this compound upstream, the plant saved money every quarter on dye. Pulp mills, leather tanners, and even oil refineries add it to prevent gunk from building up on equipment. That helps cut maintenance costs, downtime, and unnecessary waste.
Painters and construction workers might think about this compound only when cleaning brushes or mixing plaster, but its impact goes deeper. It prevents scale in boilers and helps process foods by clarifying juices or acting as a preservative in jarred goods.
Agriculture and Environmental Impact
Farmers get a boost from this compound when they spray it on crops or use it as a feed additive. It helps plants absorb important minerals and can soften irrigation water. Environmental engineers, aiming to clean up old industrial sites, use this compound to fix soil contamination—its binding power locks up heavy metals, so they don’t get into the food chain.
I’ve helped test lakes for pollution, and the presence of this compound can reveal whether a factory nearby cares about its waste streams. Regulations keep a tight leash on discharge levels, shielding waterways from harm.
Improving Life, One Small Step at a Time
From a bag of shredded cheese to big water treatment plants, the applications of this compound shape work, health, and daily comfort. These quiet contributions rarely grab attention, but they create a thread connecting different aspects of modern living. Managing dosages and handling waste require careful oversight, but the benefits—cleaner clothes, safer water, and protected crops—make it hard to imagine today’s world without it.
Understanding Product Risks and Storage Necessities
Anyone who has spent time managing storage for chemicals or sensitive goods knows just how quickly simple negligence can create a problem. Even everyday products bring their own risks and quirks if you let a bag tear open, mix it with something it shouldn’t touch or let the temperature wander off course. Accidents happen fastest in stores and stockrooms that skip clear rules for products. The label tells a story — fire hazard warnings, health cautions, expiration dates — and all those need to steer every step of handling and storing a product.
Temperature and Humidity: Why Consistency Matters
Getting storage temperature right isn’t just about meeting codes. I’ve seen firsthand what happens to sensitive materials when stacks sit in the wrong part of a warehouse. Products break down, liquids separate, powders clump up. Keeping goods in dry conditions where humidity stays low prevents molds, caking, and unexpected chemical activity. Set a product down near furnaces, vents, or where sun pours in, and changes start quietly. Good practice means keeping an eye on thermostats and making sure sunlight stays away, especially since even a few degrees’ shift can cut shelf life or trigger odd smells and color changes. Many companies use data loggers to catch these small shifts before they become big problems.
Separation and Cross-Contamination
Certain chemicals react badly when stored near each other, and sometimes that danger isn’t obvious unless you’ve handled a spill yourself. For example, stacking acids beside bases, or letting any flammable product share space with oxidizers, courts trouble. I once saw a minor mix-up balloon into a full evacuation when two poorly labeled drums wound up in the same alcove. Flammable or volatile items deserve separate cabinets—often metal, sometimes ventilated—while food and drink never belong nearby. This may sound strict, but it saves time, downtime, and expensive cleanups. Training staff to spot and report misplacement makes a big difference.
Labeling and Inventory
Every bottle, can, or package needs a clear, untouched label. Faded, torn, or hand-scrawled labels set everyone up for confusion, especially during a rushed shift change. An organized inventory, logged and updated daily, makes sure nothing slips through the cracks. Regular audits help spot leaks, expired product, or missing documentation before regulators do. From my experience, even the sharpest staff make less mistakes when they use digital barcodes or an online inventory portal with reminders and batch tracking.
Handling and Personal Safety
Personal safety isn’t just a checklist item — it’s the difference between regular work and a nightmare. Gloves, goggles, and proper aprons cut risks to skin and eyes, especially when measuring or pouring. Ventilation deserves attention, especially with powders, aerosols, or strong odors. A well-fitted respirator, or good room airflow, limits both airborne contact and longer-term health worries. Spills happen, and the best teams keep spill kits at arm’s reach, run drills, and know how to isolate a mess quickly. Eye wash stations and clear exit paths transform a near miss into a short break, not a disaster or a hospital trip.
Tackling Common Problems with Smart Solutions
Strong habits shape safe outcomes. Setting specific rules on stacking, rotation (FIFO — first in, first out), and routine cleaning goes beyond standards; it protects everyone and saves money. Digital alerts for upcoming expiration dates, combined with frequent checks for leaks and bulges, flag problems before products make a fuss. Training isn’t just for new hires — regular refreshers, easy-to-read SOPs, and reminders at every workbench keep safety close at hand. When teams talk through small slip-ups, rather than sweep them away, everyone learns and improves.
Leaning on Facts and Building Trust with Customers
Trust doesn’t come from slogans. Customers notice when orders arrive late, spoiled, or damaged. Reports from the U.S. Chemical Safety Board show storage mistakes still lead to dozens of incidents each year. A reputation for consistent safety and careful handling earns repeat business and peace of mind. I’ve learned that getting these basics right—every label, temperature check, and storage cabinet—sets a company apart and keeps people, property, and the environment far safer.
Understanding Purity – Why Numbers Mean More Than Just a Label
Choosing chemicals for production, research, or maintenance never stops with a familiar name on the container. On the front lines of any workplace, purity levels shape more than the end result; they decide the success or failure of a project. A lot of people ask themselves if a small number on a technical sheet really matters. From years of working alongside lab professionals and manufacturing teams, I have seen how a mismatch in purity leads to wasted time, lost batches, and expensive fixes. A chemical with 99% purity won’t solve every problem—sometimes, trace metals or water can mean disaster for sensitive reactions.
Pharmaceuticals need super high purity standards for a reason. Impurities can bring unpredictable side effects. In electronics, stray ions undermine the reliability of semiconductors and circuit boards, even when numbers look innocuous. Even for everyday cleaning agents, purity shifts the way those solutions perform and interact with surfaces.
Typical Purity Specifications Found in Industry
For basic chemicals, the bar sits at technical grade—enough to satisfy general purpose applications where side products won’t cause harm. In manufacturing, especially for food or medical supplies, specs climb to food grade or reagent grade, pushing unwanted elements into the parts-per-million or even parts-per-billion. That matters. Not every grade fits every use. Too many times I’ve seen companies lose money and time because they chased a cheap deal without reading the specification table.
That’s why suppliers have to publish clear, testable numbers for each batch leaving the dock. Traceability isn’t a sales gimmick; it’s peace of mind for the person opening that drum at 7 am. For the end user, finding a certificate of analysis for each shipment backs up the label claim, bridging trust between buyer and seller. Without it, you’re gambling with your process and your product’s reputation.
Why Packaging Size Shapes Choice and Safety
Once you settle on the right purity, the next challenge comes with the packaging. Large chemical manufacturers often offer standard sizes: small bottles for R&D benches, pails for mid-scale work, drums for factories, and sometimes even tanker loads if you’ve really scaled up. The packaging is more than just convenience. Small vials reduce risk to staff, keep air and moisture exposure down, and help track inventories. For me, a leftover five-liter jug might go to waste if the chemical spoils on the shelf. It saves money and protects workers to order just enough, in the right container.
Packaging also tells a story about the supplier’s priorities. Has the drum got a tamper-proof seal? Is there a batch number stamped on every label? Incompatible packaging invites spills, ruined product, and can put staff in direct harm’s way. I’ve seen what happens when a team tries to decant hazardous liquids from a 200-liter drum with no pump—someone gets hurt and the company faces weeks of paperwork.
Finding Solutions—Ask the Right Questions
Buyers need more than a sales pitch; they need technical answers. A real supplier can tell you not only the stated purity, but the main contaminants and how consistent those numbers stay from batch to batch. Before ordering, I always check with the end user. How much will you use in a week? Do you need glass or plastic containers? Can your storage room handle the load? The price per kilogram drops for bigger packages, but the risk grows. Safety, compliance, and shelf life all ride on that detail.
Smart sourcing means matching both purity and packaging to the job—asking for the test results and not settling for vague promises. The right supplier shares technical information, can back up every claim, and understands what happens after the box leaves their warehouse. From my experience, the difference between a job well done and a disaster usually comes down to the numbers no one checks until it’s too late.
Why Clean-Up Calls for Attention
A simple splash or spill can change the mood in a lab or workshop fast. At that moment, the question isn’t just about the mess—it becomes a matter of health and long-term safety for everyone nearby. Chemicals get into eyes, soak through skin, and leave residues on surfaces that never seem to truly go away. The true risk shows up later, maybe as chronic health trouble or a building that needs to be gutted after a single bad day. This isn’t about following a checklist; it’s about making sure those using or even passing through these spaces go home healthy.
Why Preparedness Matters
After years in spaces that mix science, trades, and day-to-day life, one lesson sticks: being unprepared for spills guarantees bigger problems. Basic supplies like absorbent pads, chemical-resistant gloves, and eye washes should never be on a wishlist. In fact, grabbing a box of baking soda or kitty litter off the shelf has saved more than a few surfaces and pairs of shoes from ruin in a pinch, but they have limits. Big spills demand more—enough ventilation, fast evacuation routes, and a phone close by for real emergencies.
Knowing the Chemical’s Story
No two chemicals behave the same once out of the bottle. Solvents like acetone vanish in the air and leave headaches or dizziness behind. Corrosives chew through concrete. Some, like mercury, can hang around in corners and under furniture for years, creating invisible threats. Safety data sheets (SDS) sound dry but store the hard facts—how to clean up, what never to mix with, and how fast to flush skin or eyes. I’ve seen situations where ignoring the SDS meant smears that looked clean but tested positive weeks later.
The Human Side of Clean-Up
Clean-up starts with people, not products. Training goes further than posters on the wall. A five-minute walk-through with a group, explaining the steps, always turns up questions and ideas that manuals miss. Telling stories about close calls makes the rules real—people remember what panic looks like, and it sticks better than lists of dos and don'ts. Regular safety drills can feel like a chore, but they shake off the fog and make the right choices automatic.
Tackling the Problem, Not Just the Spill
Some mistakes point to bigger gaps: poor labeling, cluttered workspaces, or old habits that crowd out safety. After a spill, honest talks matter more than punishments. Sometimes, workers reach for the nearest rag, skip gloves, or pour something down the drain. These choices come from pressure—deadlines, fatigue, or not understanding what’s at stake. Building a culture where people can stop and say, “Wait, this seems wrong,” saves more than any single clean-up kit ever could.
Better Tools, Better Outcomes
Easy access to proper spill kits, up-to-date SDS files, and clear waste disposal bins erases confusion. Signs don’t just warn, they guide—clear color codes and straight language help, especially for new team members or in places with lots of visitors. Regular checks on safety gear, from goggles to exhaust fans, keep small problems from growing. On-the-spot reminders work better than wearing a badge with a safety motto.
Looking Forward: Small Steps Add Up
Keeping chemical safety real takes constant focus, honest conversations, and steady upgrades to gear and training. No one wants to deal with the fallout from a bad spill or exposure, least of all the workers who trust their colleagues and equipment. Every improvement—better training, smarter layout, more open communication—shields not just the people right now, but generations to come who will use these spaces. Handling spills right isn’t just about cleaning up one mess, but building a healthy place for everyone who steps through the door.
Breaking Down the Molecule
Chemistry gets personal once you dive into the molecules that shape the industries around us. 2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride isn’t just a random set of elements tucked together. Its chemical formula—C7H3ClF4O2S—makes for a pretty unique compound. Behind every symbol there sits a purpose. Fluorine and chlorine bring more than just tongue-twisting names; they offer a combination that shows up in advanced material research and the design of pharmaceuticals meant to resist breakdown.
Numbers That Matter: Calculating Molecular Weight
Every chemist checks the math for molecular weight before moving ahead. This compound brings together carbon, hydrogen, chlorine, fluorine, oxygen, and sulfur. Let’s crunch those numbers:
- Carbon (C): 7 atoms × 12.01 g/mol = 84.07 g/mol
- Hydrogen (H): 3 atoms × 1.008 g/mol = 3.024 g/mol
- Chlorine (Cl): 1 atom × 35.45 g/mol = 35.45 g/mol
- Fluorine (F): 4 atoms × 18.998 g/mol = 75.992 g/mol
- Oxygen (O): 2 atoms × 16.00 g/mol = 32.00 g/mol
- Sulfur (S): 1 atom × 32.07 g/mol = 32.07 g/mol
Pack it all together and you hit a molecular weight right around 262.61 g/mol. Getting this number right saves time and stops mistakes before they leave the glassware.
Real-World Impact
Chemists see more than numbers; they picture how all those atoms work in the world. This compound shows up in the creation of specialty sulfonamide drugs and agrochemical agents. When I first set foot in an organic synthesis lab, compounds like this one gave me a crash course in the strict handling these chemicals demand. Anyone who’s caught a lungful of sulfonyl chloride vapor knows you cannot cut corners. The reactivity of the chloride group makes it easy to attach to a wide range of molecules, but that same reactivity means goggles—and good ventilation—can’t be an afterthought.
Safety and Environmental Checkpoint
Time and again, chemists revisit the importance of personal and environmental safety. Chlorine and fluorine in this structure often bring up tough questions about persistence, toxicity, and bioaccumulation. Several studies show worry over fluorinated compounds lasting in groundwater or soil for decades. This kicks off a search for safer production routes and cleaner ways to dispose of by-products. One step that makes a difference includes small-scale pilot reactions before jumping headlong into large-batch synthesis, catching surprises before they leave the hood.
What Comes Next
As patents expire and new uses pop up for complex compounds, research keeps chemists looking for alternatives that protect health and land. Some labs steer toward less persistent analogs or swap out risky groups for milder options. Others turn to green chemistry—thinking about atom efficiency and cleaner catalysts. It might take years for sweeping change, but momentum grows every time someone plugs safer data into an experimental plan.
Final Word
Getting the formula and molecular weight right sets the baseline for anything that comes after. The bigger challenge—the one that keeps you thinking long after the textbook is closed—is using that knowledge to make choices that hold up under scrutiny, in the lab and beyond.
Cleaning Products and Household Essentials
Walk down any grocery store aisle, and shelves brim with sprays, wipes, and soaps. Many of these rely on tried-and-true chemicals to break down grease, stains, and germs. In my own kitchen, a handful of ingredients always stand out on the back of the cleaning bottle. For example, sodium hypochlorite or hydrogen peroxide really pull their weight, disinfecting countertops and handling stubborn residues after a long night of cooking. On laundry day, bleaching agents like these help lift stains that seem impossible to tackle.
Many people don’t notice how closely their health is tied to these products. Strong sanitation can lower rates of infection and illness, keeping families safer, especially during flu season or in the wake of heavy storms and floods that leave behind bacteria.
Medicine and Personal Care
A trip to any pharmacy makes the chemical’s footprint clear. Oral rinses, wound cleansers, and topical ointments are all common sights. As someone who’s scraped a knee falling off a bike, that stinging sensation isn’t just psychological; it’s a direct sign that the substance is clearing out bacteria and lowering the risk of infection.
Doctors and nurses trust many of these chemicals in clinics and hospitals for prepping skin before procedures or disinfecting surgical tools. Public health improves when medical teams can depend on consistent, powerful sanitation methods.
Water Treatment and Public Health
Strong public infrastructure depends on more than pipes and pumps. Water facilities across cities and towns pour chemicals like chlorine or other purifiers into reservoirs. These additions knock down harmful organisms and keep drinking water safe. On road trips growing up, I noticed a faint taste in water at rest stops across regions. Many municipal systems rely on the same handful of chemicals in water purification processes. These guard against diseases like cholera and dysentery, problems that hit hardest where sanitation falters.
The World Health Organization points out that in places lacking these treatments, outbreaks are always a bigger threat. Just a little added to water makes a world of difference between health and serious illness.
Food Safety and Preservation
The journey that food takes from farms to store shelves leans heavily on chemistry. In packing plants, fruits and vegetables often get sprayed with mild disinfectants, trimming the risk of bacteria that could cause foodborne illness. Walking through markets, the produce that looks the freshest probably owes some thanks to these sprays.
Beyond that, chemicals like sodium benzoate sit quietly in cans and bottles, stretching shelf life and making sure that food stays safe through the peaks and valleys of global shipping. Some people choose organic to avoid additives, but food safety experts fall back on the undeniable record: fewer recalls and less spoiled food keep prices down and health complaints out of the headlines.
Industrial and Agricultural Impact
In factories, chemicals play a role that isn’t always glamorous yet stays essential. Workers rely on solvents to clean machines, degrease engines, and prepare surfaces for painting or coating. Even in agriculture, farmers use select treatments to fight pests and diseases, protect crops, and boost productivity. These practices lead to fuller harvests and a steadier food supply.
Critics point to overuse and environmental runoff, which can impact rivers and wildlife. Better waste management and tighter regulation can help communities limit the risks while preserving all the benefits these chemicals bring to daily life.
Understanding the Risks
Working in labs brings plenty of rewards, but each bottle on the shelf represents a responsibility. Chemicals like 2-Fluoro-6-(trifluoromethyl)benzenesulfonyl chloride pack a double punch: their value for research and the dangers they pose if someone cuts corners with storage or handling. Sulfonyl chlorides cause trouble if they escape their container, reacting with moisture and giving off corrosive fumes. Fluorinated variants heighten these hazards, so it pays to dig into their quirks.
Why Good Storage Matters
It’s tempting to tuck containers wherever there’s space, but with this sulfonyl chloride, lazy habits come back to bite. The chemical releases toxic gases on contact with water. That trait makes climate control and dryness not just suggestions, but requirements. In my first months in a university synthesis lab, I watched a simple shelving error trigger an evacuation after a reagent leaked onto an unknown wet spot. The lesson stuck: even the smallest oversight can snowball into chaos.
Long-term integrity for this chemical comes down to a few firm habits. Always pick a tightly sealed bottle, made of materials that can deal with aggressive acids. Glass works well, so long as it’s equipped with a screw cap lined with PTFE. The container belongs inside a dedicated desiccator or at least a dry cabinet. Humidity creeps in fast, so tossing in fresh desiccant packets protects your investment and your health. Clear labeling cuts confusion that could lead to mistakes, especially with rare or specialty reagents.
Thinking Through Handling
Any chemical strong enough to etch glass deserves respect. Gloves, goggles, and a full lab coat come standard. Nitrile gloves hold up against sulfonyl chlorides much better than thin latex. A chemical fume hood, not just an open window, keeps fumes from lingering and irritates fewer coworkers. Back in one industrial internship, I watched a technician work outside the hood “just for a minute”—that ended with an eye-watering incident for half the floor.
Working with small quantities lowers risk, yet spills still happen. Having calcium chloride or another effective drying agent on hand allows for quick control without a scramble. Never let clean-up supplies drift out of reach; emergencies punish complacency.
Disposal and Accident Response
Waste demands equal care. Pouring leftovers down the drain pollutes far more than air. Contact with water produces dangerous hydrogen chloride gas and fluorinated byproducts, which local water systems struggle to filter out. Collect spent material in a labeled, sealed container, separate from standard halogenated waste. Professional disposal services cost more up front, but regulators fine far harsher for careless dumping.
If skin contact occurs, strip gloves immediately and flush the affected area with water for at least fifteen minutes. Splashes in eyes deserve swift rinsing and immediate medical help. Document every incident both for safety records and to help identify trends that hint at larger workflow problems.
Setting the Right Culture
Precaution should never come down to personal heroics. Shared lab environments thrive on peer reminders and clear policies. Newcomers benefit most from stories of close calls rather than scare tactics. In my experience, the most successful teams treat every transfer, every bottle opening, as a deliberate act, not a mindless task. Vigilance paired with respect for the chemical keeps everyone safer, helps research progress, and makes going home in one piece a sure thing.
Understanding the Risks in Real Work Environments
Many people I know in manufacturing and lab work have stories about spills, surprise reactions, or awkward moments learning to gear up. These stories usually share a common thread: somebody trusted their luck, skipped the gloves, or “just needed to check something quick.” That’s all it takes for irritation, confusion, or lasting damage. Nobody sets out hoping for a problem. Unfortunately, forgetting one step with the wrong product changes the story for everyone. Facts tell us the same thing. The Bureau of Labor Statistics shows that skin and eye injuries still make up a big part of workplace incidents across the United States. Most of those cases could have been stopped with the right protection—simple, not fancy.
Core PPE for Chemical and Industrial Products
Every product, especially chemicals and powders, comes with its own risks. Basic safety calls for covering the skin, eyes, nose, and mouth. At the top of the list are safety goggles or a full-face shield to block splashes, powder bursts, and fumes. Never trade regular glasses for real goggles. Glasses leave open gaps. A good experience is walking away from a splash unharmed because the right goggles made a seal. Simple gloves—like nitrile, neoprene, or whatever works with the chemical—keep skin safe. Ordinary latex rarely holds up against strong solvents or acids.
Lab coats or chemical-resistant aprons block splatters and spills from reaching clothes. Open sleeves and collars welcome in dust and drips; it feels better to wear fitted cuffs and closed necklines. Strong shoes that cover the whole foot—no mesh or sandals—cut down the risk if something lands down low. In dusty situations, a properly fitted N95 or P100 respirator prevents fine particles from entering the lungs. Gases and stronger chemicals might need a full-face cartridge mask. It’s tempting to reuse masks, but each one has a limit for safe use. Respirators also demand fit testing, a step some workplaces ignore to save time.
Work Practices and Personal Routines Matter
PPE only works when people use it from the start and don’t drop their guard later. No gloves for “just a minute” leads to trouble fast. Safety looks like checking that eyes and hands are covered before even opening a new container. Keep drinking cups and food far from the workbench, since even a dusting on a rim can cause real sickness. Remove and store protective gear away from break rooms or home spaces—carrying chemicals on shoes or sleeves happens more often than some think. Wash hands with soap right after finishing, not just with a quick water rinse.
Real Solutions: Training and Availability
Good training and clear labels give everyone the facts up front. A one-time safety meeting loses its power if new hires never hear it. Signs and checklists posted at eye-level remind even busy workers that safety steps aren’t optional. Supervisors and old-timers model good habits—everybody follows their lead without much fuss. Supplies like gloves and replacement masks belong within arm’s reach, not locked in a distant office. If a company makes PPE easy to get, people rarely skip it.
Everyone wants to end the workday as healthy as they started it. Having the right equipment on-hand makes that much more likely. Real-world safety comes from regular people, not just policies. Small decisions—every shift, from every team member—add up to big results nobody regrets.
Understanding What You’re Getting
Some folks see a jar with a chemical label and just assume every grain or milliliter inside matches what’s printed. That’s not always the case. I’ve seen surprises spring up during projects when the chemical purity or grade looked perfectly fine on the invoice, but the reality didn’t match the expectations. Purity isn’t just a matter of pride for suppliers. For research labs, manufacturers, and anyone running quality checks, purity reflects the difference between success and a false read—or a wasted batch.
There are real stakes here. Imagine running reactions in an academic lab with something called “analytical grade” on the jug. If that’s truly above 99.5% pure, your experiment gets a clean slate. Drop to “technical grade,” usually under 90%, and you start guessing which impurity crashed your process. Some industries, like electronics, get only a whisper of tolerance before a contaminated reagent means a major loss.
Don’t Skip the Fine Print: What Grade Means in Practice
“Grade” may sound like something for teachers, but it’s a shorthand for how much trust you can put in the contents. Pharma companies pay more for pharma-grade chemicals because a stray trace of something else could affect people’s health. Food producers demand “food grade,” a step above industrial or agricultural chemicals. Sometimes, people get caught by the subtlety—two bottles with similar names but wildly different specifications. That’s more common than we’d like to admit.
Without this information, risk snowballs. Just ask anyone who's worked with “lab grade” solvents and seen the impact on chromatography results. It’s not only about purity; the process used during production can bring other compounds with similar weights or boiling points. Once, early in my career, ordering a less expensive solvent ruined several columns. Nobody wants to relive that, and it's a hard lesson in ignoring the details.
Packaged for Precision—or Not
Packaging doesn’t just mean how pretty the bottle looks. Bulk orders can save money, but huge barrels may not store well, might degrade or contaminate the substance, or make measurement trickier for smaller operations. Some grades come in sealed ampoules for pharmaceutical use, each batch marked and traceable. Others arrive in industrial drums. The wrong container can mean lost product, wasted chemicals, or serious safety issues—leaks, spills, reactions with air or moisture. One leaky drum of an acid or base in the wrong room can halt the whole day and put people in danger.
Digging for Information and Demanding More
Suppliers offer technical data sheets, but not all keep theirs updated, and some hide behind vague terms. A short phone call can save hours: “What’s the actual purity? Which impurities have you tested for?” Don’t be shy asking about recent batch certificates of analysis. These prove what’s inside matches what’s sold. If someone can’t produce solid data, that’s a red flag.
The broader struggle has roots both in regulation and the fragmented supply chain. Strict agencies, including FDA and REACH, keep rules tight in some sectors, but not everywhere. Buyers end up navigating a maze of certifications—ISO, USP, ACS, and others. Sometimes, competition or budget pressure pushes folks to accept less-than-complete information, and the fallout lands on the floor of their labs or plants.
Raising Expectations and Demanding Quality
Pushing back on missing or unclear data isn’t overreacting. It’s common sense. Quality, safety, and reputation rely on these details. Whether brewing beer or developing new materials, clear, reliable specifications matter. In this industry, “good enough” can turn quickly into “not good at all.” The best safeguard is a critical eye, a handful of direct questions, and a willingness to walk away from unknowns.