2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole: A Deep Dive into Chemistry and Application
Historical Development
Chemists began exploring thiadiazole derivatives in the first half of the twentieth century, searching for molecules with strong bioactivity. Building on this curiosity, researchers synthesized 2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole as part of a wave of innovation driven by the pharmaceutical industry's need for unique scaffolds. Early patents point to intense exploration in the mid to late 1900s, as medicinal chemists recognized the particular importance of incorporating both an amino group and a trifluoromethyl group onto a heterocyclic framework. The thrust for new agrochemicals and drug candidates gave this molecule its first launch pad, showing how close observation of natural molecular structures leads to versatile compounds that continue to fuel research.
Product Overview
2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole is a nitrogen and sulfur-containing heterocycle, recognized for its ability to serve as an intermediate in both pharmaceutical and agricultural chemistry. Commercial catalogs list it under CAS number 328-84-7, marking it as a valuable building block, suitable for entry into a wide range of chemical transformations. Its small size, reactive sites, and existing functionalization make it attractive for laboratories working on advanced syntheses, custom molecule production, and as a diagnostic handle in molecular biology. Chemists appreciate how this structure lets them test new theories without building every component from scratch.
Physical & Chemical Properties
This compound presents as a crystalline solid, generally white to light yellow, depending on purity and storage. Its melting point falls between 151°C and 154°C, a range that responds to slight impurities or solvates picked up during handling. It dissolves best in polar organic solvents like dimethylformamide or DMSO, enjoyed for the pronounced solubility that aids in reaction workups and purification steps. On the chemical front, the thiadiazole ring stabilizes the molecule against hydrolysis and oxidation, while the trifluoromethyl group increases both lipophilicity and resistance to metabolic breakdown. This gives chemists a reliable starting point for reactions that demand a tough core structure.
Technical Specifications & Labeling
Suppliers of 2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole list purity grades, typically ranging from 97% up to 99%. Analytical data, including HPLC retention times and NMR spectra, back up these claims, giving buyers confidence that what is listed matches what arrives. Most packaging features hazard labeling, signaling respiratory or dermal exposure risks. Labels include CAS numbers, batch identification, gross and net weights, and supplier contact details, reflecting the importance of traceability and safety for every batch. Good logistics start with clear information, and in the lab, I have seen how even a missing detail can halt experiments for days.
Preparation Method
Most routes to this thiadiazole rely on cyclization reactions, starting from simple thiosemicarbazide precursors. By introducing trifluoroacetic anhydride in the presence of mild base, the reaction closes the ring and installs the trifluoromethyl group. Yield optimization focuses on low temperatures, solvent choices like acetonitrile, and careful extraction protocols. Chemists often purify the compound by recrystallization or column chromatography, sometimes needing to squeeze the best solid from a mix of solvents to achieve analytical quality material. It takes practice and a little intuition to know exactly when the product reaches peak purity.
Chemical Reactions & Modifications
The molecule's amino group opens a menu of possibilities for further chemistry. It reacts readily in acylation, sulfonation, or alkylation steps, letting researchers tailor properties for targeted applications. Scientists attach fluorescent tags, swap in bulkier groups, or use it as a linchpin for linking other pharmacophores. The trifluoromethyl group resists many environmental degradations, anchoring the molecule through tough transformations. In practical terms, adding anything at the 2-position dramatically alters biological activity, as even a small change here rewires how the molecule interacts with biological proteins.
Synonyms & Product Names
Chemists might refer to this compound as 5-(Trifluoromethyl)-1,3,4-thiadiazol-2-amine, or as trifluoromethylaminothiadiazole. Wholesale and research suppliers may shorten the label to TFMTD or call it by its CAS number. Having multiple names floating around can cause confusion in search results or hazardous materials inventory, so strict documentation helps prevent duplicate orders or misidentified stocks. Any laboratory with a regular inventory of specialized reagents keeps a cross-reference (including CAS numbers), making audits and regulatory compliance much easier.
Safety & Operational Standards
Inhalation, skin contact, or accidental ingestion all carry health risks with this compound. Material safety data sheets from leading vendors list precautionary measures: gloves, goggles, fume hoods, and proper skin coverage. Laboratories demand spill kits and rigorous waste handling when dealing with thiadiazoles, given both the unknowns in toxicity and the environmental persistence of fluorinated compounds. Experienced researchers train junior staff on real incidents – accidental spills or splash exposures – highlighting that strict protocols save both time and health. Regulators now expect seamless documentation, from receiving the bottle to the final destruction of residues.
Application Area
Drug discovery projects value 2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole for its ability to act as a core scaffold in anti-infective, anti-inflammatory, and anti-cancer compounds. Agricultural scientists use it as a backbone for engineering pesticide candidates, relying on the chemical's metabolic stability to withstand field conditions. Analytical chemists consider it a strong choice for tracer development, leveraging the distinct NMR and mass signals that a trifluoromethyl group delivers. Having worked in medicinal chemistry, I appreciate how a single building block can unlock divergent pathways, letting project teams rapidly cycle through analog generations in pursuit of better properties.
Research & Development
New generations of this compound spring from collaborations between synthetic chemists and computational modelers. Researchers take experimental SAR (structure–activity relationship) data, fold it into molecular models, and predict which substitutions might elevate potency or reduce toxicity. Analytical chemists push the boundaries by developing new methods for rapid purity checks and bioavailability screening. This practical approach produces tangible leads rather than theoretical papers. Smaller academic groups often publish on reaction innovations, driving industry to re-examine methods for cost savings or greener processes. My own projects benefited from such cross-pollination, as every lab brings its unique strengths to the table.
Toxicity Research
A persistent question for any molecule with a trifluoromethyl group revolves around safety. Studies in vivo and in vitro assess mutagenicity, acute toxicity, and metabolic breakdown. Early data flag skin and respiratory irritation, while more recent animal studies look for organ-specific toxicity and bioaccumulation concerns. Environmental toxicologists point out the hardiness of the thiadiazole core and the slow breakdown of the fluorinated group in soil and water, which compels regulatory agencies to consider long-term impact as well as short-term harm. Direct experience reviewing toxicology data reminds me that transparency and rigorous peer review often weed out risky compounds at an early stage, steering research towards safer alternatives.
Future Prospects
Continued research aims to modify the thiadiazole core for both therapeutic and crop protection uses, taking advantage of metabolism-resistant fluorinated motifs and the versatile amino group. Advances in reaction automation and greener chemistry could drive down production costs, making these specialty compounds affordable for mid-scale applications. Computational chemistry is speeding up structure prediction for new analogs, while regulatory guidelines on fluorinated residues prompt greater focus on degradable analogs. Industry leaders are investing in collaborative research, recognizing that effective and safe chemistry requires both technical mastery and proactive safety research. My experience tells me that the clearest path forward extends from open data, creative chemistry, and continuous vigilance for both health and environmental impacts.
A Versatile Building Block in Modern Chemistry
2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole usually pops up in technical conversations among chemists, but its story reflects a wider trend in how the world thinks about developing new medicines and materials. This compound looks unassuming. Yet, in research labs, its value stretches across pharmaceuticals, agrichemicals, and materials science, touching daily life in ways most of us never notice.
Fueling Innovation in Drug Discovery
The backbone of many modern medicines depends on small tweaks to molecular structures. With its trifluoromethyl and thiadiazole groups, this compound offers a gateway to fresh pharmaceutical candidates. Medicinal chemists often seek out structures like this because the trifluoromethyl group can change how a molecule interacts inside the body—making drugs stay longer in the bloodstream or attach to their targets more tightly. Several research papers highlight that adding such groups can lead to better absorption in the digestive tract or better resistance to breakdown. Based on my own experience helping students sift through patent journals, I’ve seen its footprint in cancer drug candidates, antifungal tests, and experimental treatments for inflammatory diseases. Scientists aren’t working in isolation here; international collaborations use this thiadiazole as a core piece when screening huge libraries of potential medicines.
Shaping Modern Crop Protection
Farmers also benefit from advances in chemical design. Crops face constant threats from blights and insects, and the search for new pesticides will not end soon. In this field, researchers have plugged 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole into larger molecules to make pesticide candidates with unique ways of fighting fungi or insects. Adding a trifluoromethyl group isn’t just a chemistry trick; it often ramps up the power of the final product and improves its stability in the field, helping chemicals last through weather or sunlight. In agricultural patents from the last decade, this compound forms the root of fungicides that don’t fit into older chemical classes. I grew up in a farming community, and the stories from those who handle crop loss remind me that incremental tools like this matter, especially now that pests adapt to traditional sprays faster.
Advanced Materials and Beyond
Beyond medicine and farming, this thiadiazole pops up in more experimental corners—electronics and smart coatings, for instance. Engineers and scientists appreciate its ability to add flexibility and unique electrical properties to polymers and dyes. In recent years, high-performance paints or films that need specific resistance to heat or chemicals have relied on molecular ingredients from this family. Scanning the booths at a materials science conference last year, I found several startup founders showcasing prototypes that blend new fluorinated thiadiazoles into sensor materials and lightweight circuit parts. These breakthroughs might not splash onto the front page, yet the results slowly shape how technology reaches consumers, through better gadgets and more reliable medical devices.
What Matters Most
Real progress with 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole does not spring from fancy jargon. It comes down to hard-won expertise, testing, and a willingness to look beyond old formulas. The road forward depends on access to up-to-date data, cross-field cooperation, and clear safety protocols—especially with more pressure for “greener” chemistry. Building bridges between pharmacists, farmers, and engineers will push these compounds from lab curiosity to useful innovation in everyday life.
Understanding the Molecular Formula and Structure
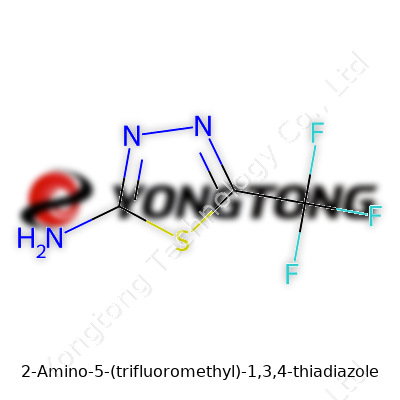
2-Amino-5-(trifluoromethyl)-1,3,4-thiadiazole goes by the molecular formula C3H2F3N3S. Its backbone contains a five-membered heterocyclic ring, which sets the stage for unique properties. On one end sits an amino group (NH2), which pulls in attention for those working in pharmaceutical or agrochemical synthesis. At the fifth position, a trifluoromethyl group (CF3) hangs off the ring, pushing the molecule into a different level of chemical behavior. Take a pen and sketch: start with the ring — S at position 1, two nitrogens at positions 3 and 4, carbon at 2 and 5, attach NH2 to carbon 2, and CF3 to carbon 5. It’s not just a jumble; this arrangement changes how the whole molecule interacts with surroundings, especially water, lipids, or target enzymes.
Why This Structure Matters in Real Life
In the world of chemical building blocks, small changes in structure spark big shifts in function. For years, I worked with heterocyclic compounds in crop protection and drug discovery. The thiadiazole ring attracted our attention for its stability and versatility. The amino group turned it into a handle for further modifications — you can swap it out or link new chemical pieces. That CF3 group, with its stubborn electronegativity and bulk, blocks some reactions but also improves things like chemical stability, metabolic resistance, and ability to cross cell membranes. Medicinal chemists often lean on these patterns to design molecules that last longer in the bloodstream and evade early breakdown in the liver. For folks designing fungus killers or herbicides, this molecule structure stands out. Trifluoromethyl groups often ramp up bioactivity, especially where you need molecules to stick around in tough outdoor conditions. Many patents from the last decade focus on rings like this for disease and pest management because they mix toughness and targeted action.
Challenges and Solutions in Handling and Application
Not every benefit comes without a hurdle. Manufacturing these kinds of compounds calls for good containment, because the trifluoromethyl group can release stubborn, sometimes toxic byproducts if things go sideways in the lab. In my own experience, solid exhaust filtration, closed transfer systems, and full protective equipment did more than check boxes — they kept colleagues healthy. Waste management rules keep tightening due to concerns over fluorinated byproducts, which linger in soil and water. Modern methods now favor greener chemistry. Catalytic direct fluorination using milder reagents or electrochemical routes limit environmental impact. Good lab stewardship involves double-checking downstream effects before scaling synthesis.
Real-World Impact and Forward Thinking
It’s easy to overlook basic research on small molecules like 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole. Yet this ring appears in prescription antifungals, new weed controls, and promising anti-infectives in early-stage trials. The structure stands as a sturdy launching pad for both large-scale commercial products and modular drug discovery. For chemists, this backbone gives flexibility to tune target fit and pharmacokinetics. For industries hoping to break resistance cycles or cut environmental footprint, pushing further on sustainable synthesis matters. In the years I’ve watched the sector, open data sharing and honest environmental auditing have pushed both safety and innovation forward. Teams that cross specialties — synthetic, biological, and environmental chemistry — solve problems faster and avoid old mistakes. In the end, the structure of this molecule shapes more than just its chemistry: it guides safety, policy, and the pace of progress in both medicine and agriculture.
Real-Life Stakes with Storage
Chemical storage isn’t just another back-room chore nobody cares about. I’ve watched smart people cut corners, throw bags or bottles on any nearby shelf, let the temperature drift, and hope for the best. A few years ago, a friend in the lab next door lost a chunk of research—six months of work—because his compounds absorbed moisture overnight. Packing up dusty remains and filling out insurance reports doesn't bring that time back. Even now, every time I tighten a cap or check a label, his story feels like a reminder from the universe: small stuff matters.
Keeping Temperature and Humidity in Check
Dry goods soak up moisture once the container gets cracked open. Even if the label says “stable at room temperature,” I’ve seen hydroscopic powders clump up on rainy days just from opening the jar on the bench. A cool, dry cabinet shields much better than a shelf above the sink. Lots of folks use a desiccator for powders—doesn’t need high-tech solutions, just basic keep-dry packs and a check once a week.
Mixing up bottles with similar names happens more often than anyone admits. Heavy labeling—date, initials, concentration—keeps everyone honest, especially in crowded fridges or shared storage. For liquids, vapor-tight seals aren’t just for show. The sharp stink of solvent leaking out of a loose cap tells you exactly where someone got lazy.
Light, Air, and Their Surprises
Sunlight isn’t always your friend. Plenty of compounds break down simply from UV exposure. I've left samples by a window, only to find unexpected colors thanks to light-induced reactions. Amber glass, foil covers, or keeping stuff out of direct light easily blocks that risk. For air-sensitive reagents, special cabinets pumped full of inert gas or just a steady nitrogen blanket change the game completely. Without them, expensive stock evaporates—literally and figuratively.
Handling: It’s Not Just About Gloves
Wearing gloves, goggles, and a coat goes without saying—but not everyone does. Too many folks grab and go, not stopping to grab a scoopula instead of dumping powder out of the bottle, spreading contamination. Keeping a log of what came out, who took it, and whether the bottle got closed right means fewer accidents. I worked somewhere once where people scribbled names with dry-erase markers on almost every bottle; it reduced “mystery spills” to basically zero.
Getting Rid of Waste and Spoiled Product
Discarding old or decomposed materials seems obvious, but old vials fill up drawers fast. Pouring scrap in regular trash might sound like a shortcut, but the legal headaches from an environmental accident—or the sharp sting of a chemical burn—stick around far longer than the few seconds saved. Following disposal guidelines, using marked bins, and jotting down waste in a logbook backs up your story if regulators or safety officers come by for a surprise check.
What Actually Works?
Improvement grows from small habits. Stick a temperature gauge in your storage, maybe tack a checklist beside the cabinet, and talk openly about mistakes. Rethink labels—is everything easy to read from a distance, even in a hurry? Shield fragile stuff from light, screw those caps down tight, and keep a written record. None of these tricks require huge budgets, but every one pays off before long—in safety, in fewer lab scares, and in work you can trust.
What Makes This Chemical Noteworthy?
Handling chemicals always brings a bundle of questions, especially unknowns like 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole. This compound doesn’t carry a familiar name like household bleach or acetaminophen, but its structure—with a trifluoromethyl group—calls for attention. That trio of fluorines tends to boost chemical stability and lipophilicity, meaning it could stick around in the environment or in your body longer than you’d like. Sometimes, the presence of fluorine atoms links with toxic effects that can show up well after direct contact. Experienced researchers know that ignoring these kinds of features opens the door to long-term headaches.
Toxicity and Hazards: What Has Science Found?
Published safety data on this molecule is limited, but the building blocks tell their own story. Many 1,3,4-thiadiazole derivatives see use in labs working on pesticides, antimicrobial compounds, and other pharmaceuticals. A handful of these compounds show toxicity on contact or inhalation, causing symptoms ranging from skin irritation to organ-specific problems if the dose climbs. Perfluorinated and trifluoromethyl compounds, in particular, have drawn scrutiny because they sometimes resist breakdown, leading to bioaccumulation and disruptive health effects—including liver enzyme disturbances and possible links to cancer with long-term exposure. I've seen several reports where workers dealing with fluorinated chemicals developed unexplained ailments until someone tracked exposure levels over weeks, uncovering the culprit. That stuck with me. Just because the compound lacks dramatic warnings doesn’t mean it’s safe at scale or over time.
How Are Exposure Risks Handled?
Laboratories and factories, where thiadiazole derivatives are made or handled, set strict rules for a reason. Gloves, goggles, fume hoods, and well-maintained ventilation work better than makeshift solutions. Organizations like OSHA and NIOSH suggest reviewing Safety Data Sheets and using air monitoring devices, especially with chemicals sporting multiple fluorine atoms or sulfur-nitrogen bonds. I’ve found that one shortcut in the lab—a forgotten glove swap, a fleeting “it’s only a dust”—can mean days of discomfort or worse for coworkers. So, even if published profiles downplay acute toxicity, daily discipline matters most.
Environmental and Public Health Impacts
The world already faces lasting trouble from perfluorinated and highly fluorinated chemicals. Rivers and groundwaters near chemical sites tested positive for these persistent compounds; even trace levels draw alarm bells among ecotoxicologists. If manufacturers release waste containing 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole or its byproducts without proper treatment, wildlife and local communities could shoulder risks for generations. Studies in environmental science journals keep tracing new links between trace chemical exposure and subtle disruptions in fish, amphibians, and people who rely on local water.
Building Safer Practices
Full transparency about exposure risk, reporting any spills quickly, and keeping thorough records help prevent trouble before it starts. Workers and researchers deserve safety briefings beyond boilerplate warnings. Proper disposal programs and investment in greener chemical alternatives both pay off in the long haul, shifting from firefighting to genuine stewardship. Sharing findings, supporting toxicology research, and demanding tighter industry standards moves everyone toward healthier workplaces and communities. The lessons I learned over two decades stress that early caution beats crisis management every time.
Practical Advice for Today
If you ever need to handle or transport 2-amino-5-(trifluoromethyl)-1,3,4-thiadiazole, never let routine dull your respect for unknowns. Request or review an up-to-date Safety Data Sheet, use the right personal protective equipment, and don’t trust luck to keep you safe. Chemical hazards rarely give fair warning—they catch out those least prepared for them.
Purity Grades: Why They Matter
Picking out a chemical or ingredient for work or home use, I always stop to check the purity grade. Quality makes a big difference, and it’s not just about getting what you pay for. Purity grades range from technical, lab, to reagent or pharmaceutical grades. For instance, technical grade chemicals show up in cleaning products or bulk industrial applications. They don’t need to be spotless, just good enough for heavy-duty jobs. Laboratory and reagent grades kick it up a notch, offering higher purity for precise experiments in research, diagnostics, or food testing. Pharmaceutical grade stands out as the cleanest, with standards high enough for use in medicine and personal care.
Regulators watch over these grades closely. Organizations such as the US Pharmacopeia (USP) and the American Chemical Society (ACS) set the bar high for anything that touches people directly. Standards aren’t meant to be intimidating—they protect the consumer, the scientist, and the worker by keeping contaminants at bay.
I’ve handled everything from technical grade cleaning agents to pharmaceutical compounds, and one thing stands out: the higher the purity, the stricter the storage and handling. That means any slip in quality control can cause batches to drop a grade, and that loss stings in wasted money and time. It's not just about having a clean product, either. Even a pinch of the wrong contaminant can throw off an experiment, threaten patient safety, or cause a manufacturing shutdown.
Packaging Sizes: More Than Convenience
Packaging says a lot about both the product and the people using it. Small bottles—30 grams, 100 grams, maybe up to 500 grams—appeal to labs running small tests or hospitals preparing single treatments. These sizes also help reduce waste, which I always appreciate once I remember the hassle of disposing of chemicals safely.
Large-scale users aren’t interested in tiny containers. They want drums, pails, or even IBC totes of 25 kilograms, 200 liters, or more. Industrial buyers think in tons, not milligrams. Storing these volumes calls for strong packaging—durable plastics or steel with clear labels detailing grade, warnings, and expiry dates. For high-purity needs, manufacturers often use amber bottles or airtight bags to keep light and moisture away, keeping the product stable.
Smaller pack sizes might cost more per ounce, but often pay for themselves by cutting storage headaches and reducing risk of contamination. I’ve found that working with the right size cuts down on accidents—less product sitting open, less chance of spills or mix-ups. That simplicity can be the difference between a smooth shift and a dangerous scramble to handle an unexpected leak.
What Can Be Improved?
Manufacturers could offer clearer labels that help users pick the right grade. Technical jargon fills the fine print, making it hard for people without a chemistry background. More straightforward guides would make shopping easier and avoid pricey mistakes. Offerings could also be more flexible, with customizable sizes for buyers stuck between tiny research packs and massive industrial shipments. I’ve seen companies forced to buy more than they need because their ideal size doesn’t exist—and that creates waste.
Getting input from users—everyone from lab techs to warehouse managers—can drive smarter packaging decisions. Connecting with workers who handle these products daily uncovers needs for better safety features, like tamper-proof seals or child-safe caps. Simple changes add up in preventing accidents and keeping products fresh.
Moving Forward
Bottom line, purity grades and packaging go hand in hand. They're not afterthoughts—they shape safety, efficiency, and even affordability. Being clear about these options and asking for real feedback leads to better products all around. It’s about people getting exactly what they need, no more and no less, with minimal fuss and maximum safety.