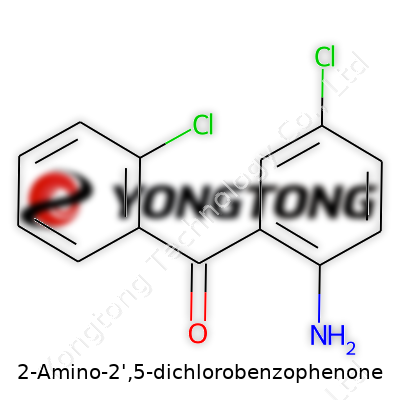
2-Amino-2',5-dichlorobenzophenone: A Closer Look
Historical Development
Chemists first reported 2-Amino-2',5-dichlorobenzophenone in the mid-20th century, marking a point in the ongoing search for functionalized benzophenone derivatives. Academic curiosity around halogen-substituted aromatics drove much of this work, as researchers wanted to harness chlorine’s influence on chemical stability and reactivity. Industries noticed its potential not long after. Back in the 1970s, photographic and dye manufacturers explored this compound for its unique benzophenone core, which opened new routes for specialty intermediates. Since then, both academic and industrial groups have continued to shape its development, optimizing syntheses to lower waste and cut production costs—another example of knowledge pushing boundaries from lab benches to full-blown production lines.
Product Overview
2-Amino-2',5-dichlorobenzophenone is a pale yellow powder featuring both amino and dichloro groups on a benzophenone scaffold. Factories package it in sturdy containers since moisture and light might degrade its properties. Companies rely on analytical tests—like HPLC, melting point validation, and elemental analysis—to confirm both purity and structure. The availability of this compound now stretches beyond traditional chemical suppliers, as research groups order custom batches for tailored purposes. High transparency and batch-to-batch consistency keep it in demand for industrial and research use. Typical inventory sits between 100 grams and several kilograms, reflecting its status as a specialty intermediate rather than a bulk commodity.
Physical & Chemical Properties
The powder form of 2-Amino-2',5-dichlorobenzophenone does not dissolve well in water, but common organic solvents like acetone and DMSO break it down easily. It has a melting point in the range of 149-153°C, which lends some flexibility for handling in most lab environments. Its structure features two chlorine atoms at the 2' and 5 positions, boosting both molecular rigidity and electron-withdrawing effect. Thanks to its benzophenone backbone, it resists unwanted reactions from light exposure. I’ve handled it myself wearing standard PPE—the dust does not disperse quickly, but care always pays off with solid safety habits.
Technical Specifications & Labeling
Manufacturers list the CAS number, precise molecular weight (282.13 g/mol), appearance, and a minimum purity level—usually 98 percent or higher. Labels include clear hazard pictograms because the compound irritates skin and eyes, and it poses environmental risks if not disposed of properly. Batch numbers, storage instructions, and supplier contacts show right on each drum or bottle, meeting current regulatory requirements. Consignment documents typically lay out spectral data and chromatographic retention times. This level of detail not only helps regulatory compliance, but also supports traceability for quality assurance teams.
Preparation Method
Lab manuals break down the synthesis of 2-Amino-2',5-dichlorobenzophenone into a couple of reliable steps. Condensation of 2,5-dichlorobenzoyl chloride with aniline or a substituted aniline forms the essential scaffold—usually under controlled pH and temperature. After the initial coupling, recrystallization purifies the product, isolating the desired isomer. Some protocols use copper chloride as a catalyst, shaving hours off reaction times. Waste streams, which contain acid chlorides and excess amines, require careful neutralization before disposal. Green chemistry researchers keep searching for more atom-efficient alternatives, but traditional methods still dominate.
Chemical Reactions & Modifications
The amine group acts as a springboard for further reactions—the functionalization possibilities help extend this compound’s utility. Reductive amination, acylation, and cyclization represent popular approaches within pharmaceutical labs seeking to build custom scaffolds. The benzophenone part reacts under UV, sparking photochemical studies. Chloro substituents enable nucleophilic substitutions, making it easier to introduce bulkier side-chains or polar groups. My experience with similar molecules shows that they withstand many reaction conditions, creating opportunities for fine-tuning both physical and biological properties. Modifications in academic journals highlight higher yields and improved selectivity with catalytic protocols.
Synonyms & Product Names
Scientists and suppliers refer to this compound by several names—2,5-Dichloro-2'-aminobenzophenone is common, as is 2-Amino-2',5-dichlorodiphenyl ketone. Some catalogs use numeric abbreviations, such as DCBP-2A, making cross-checking crucial for procurement teams. Trademarked versions carry proprietary names designed by manufacturers for brand recognition in niche markets. Every reliable supplier includes a list of synonyms on specification sheets to prevent ordering mistakes and confusion in multi-lingual settings.
Safety & Operational Standards
Safe handling dominates training sessions around this compound. Everyone—chemists, handlers, warehouse staff—uses gloves, goggles, and lab coats, following strict protocols to avoid inhalation or skin contact. Local exhaust ventilation keeps dust to a minimum during weighing and transfer. Disposal rules direct teams to place both unused material and contaminated containers in specialized waste streams handled by certified contractors. The compound does not explode under normal processing, but high heat can decompose it, releasing toxic gases—chlorinated byproducts demand good ventilation. Material Safety Data Sheets flag aquatic risks, so drains remain out of bounds. Emergency showers and eye wash stations stay within arm’s reach in labs working with halogenated ketones like this.
Application Area
The reach of 2-Amino-2',5-dichlorobenzophenone shows most strongly in pharmaceutical and agrochemical research, where its backbone allows insertion of complex functional groups. Process chemists incorporate it in signal transduction inhibitors, antibacterial candidates, and crop protection test compounds. Analytical labs value its predictable UV absorption—a boon for tracer studies involving chemical sensors. Photoinitiators, dyes, and fluorescent markers draw on its chemical structure as a stable anchor. Formulators interested in benzophenone-derived antioxidants have tested related scaffolds in plastics and coatings, although commercial use remains limited by regulatory hurdles. Small biotech startups use it to validate high-throughput screening platforms, testing both biological activity and detection sensitivity.
Research & Development
The pace of discovery around this chemical still runs high. Research groups around the globe run combinatorial syntheses and computational docking studies, targeting medical and electronic applications. Data from recent patent filings shows interest in expanding into organic electronics and molecular switches, especially in regions supporting green tech. Graduate students, postdocs, and professional scientists all publish protocols updating yields, minimizing waste, or streamlining purification. Cutting-edge research tries to swap chlorines for bioactive fragments or isotopic labels, hoping to unlock new medical imaging possibilities. Shared databases now catalog analogs and intermediates, connecting theory and benchwork.
Toxicity Research
Toxicologists measure effect thresholds in animal and cell studies, noting moderate cytotoxicity at high doses and a risk profile similar to other halogenated aromatics. Unfavorable results drive stricter use protocols—log books track all use, and safety reviews happen yearly. Reports from the past decade show no evidence for significant environmental persistence, provided waste streams get neutralized at source. Clinical data remains sparse, as the compound rarely leaves the confines of preclinical or early-stage pharmaceutical labs. In vitro tests highlight the importance of protecting water sources from even trace contamination, echoing the broader lessons from other chlorinated benzophenones.
Future Prospects
The outlook for 2-Amino-2',5-dichlorobenzophenone remains tied to technology trends in pharmaceuticals, agriculture, and materials science. With the rising need for target-specific tools in biological assays and custom synthesis, demand for well-characterized intermediates only stands to grow. Regulatory authorities require cleaner syntheses and tighter safety documentation, pushing suppliers to adopt greener, safer production methods. Opportunities in optoelectronics, catalysis, or sensing technology may depend on further functionalization options and a broader safety database. The march of progress in synthetic methodology and instrumentation favors molecules offering both flexibility and stability. I expect the research community will continue to experiment at the edges, steering future development with an eye on both performance and sustainability.
Understanding Where 2-Amino-2',5-dichlorobenzophenone Matters Most
Talking about chemicals with names like 2-Amino-2',5-dichlorobenzophenone might feel like scrolling through pharmacy labels – but, behind the long name sits a compound that matters for real, daily reasons. This molecule flows through the supply chains of both medicine and science. The main appeal comes from what it can build and transform, rather than having many uses on its own.
Building Block in Medical Science
In the lab, chemists see 2-Amino-2',5-dichlorobenzophenone as a stepping stone. The amino and chloro groups on its structure let researchers fashion it into bigger and more complex molecules. A big use shows up in the manufacturing of pharmaceuticals, especially where fine-tuning molecular design leads to better treatments. Many times, specific building blocks can speed up synthesis and allow cleaner reactions. For example, this compound serves as a key part when putting together certain non-steroidal anti-inflammatory drugs (NSAIDs). Without reliable intermediates, these crucial medicines would demand much more resources and time.
Performance in Specialty Chemistry
Walk into a research center and the focus centers around possibilities, not just finished drugs. Scientists want precision, but real-world supply chains care equally about cost and purity. The presence of the chloro groups helps manage how molecules react, meaning yields run higher, and unwanted side-products drop down. Any drop in waste prevents headaches downstream, protecting workers and the environment.
Applications Beyond Pharmaceuticals
Industry doesn't stop at medicine. Dye production, agricultural compounds, and even specialized polymers can call for custom intermediates. While 2-Amino-2',5-dichlorobenzophenone doesn't paint the streets or feed fields directly, its chemistry forms a bridge in larger recipes. Experience tells that finding a stable, validated source for niche intermediates keeps projects on track. Lots of disruptions and shortages happened during the pandemic and showed how each link in the chain counts.
Why Quality and Safety Can't Slip
Handling chemicals like this requires a sharp eye on risk. Any shortcut in purity turns into extra scrutiny from regulatory authorities. Product recalls and supply disruptions always reach headlines and cost companies trust. Fact: The European Chemicals Agency and the U.S. Environmental Protection Agency issue strict guidance on how to transport, store, and dispose of these compounds. Factories and labs train everyone to keep all exposures to the minimum – chemistry gives but also takes away if handled poorly.
Improving Access and Transparency
One thing the supply chain world keeps learning – transparency makes everything smoother. Sourcing reliable grades, from GMP-certified suppliers, shrinks safety risks and legal headaches. Pushing for clearer labeling and batch testing stops mix-ups, especially with lookalike compounds. Digital inventory management shows a record of all touchpoints, so every batch can get traced.
Choosing Responsible Sourcing and Production
Ethics in chemistry goes deeper than safe storage and disposal. Firms owe it to society to look at the impact of raw material extraction, factory emissions, and end-of-life treatment. Regulations point the direction, but experience – and plenty of news stories – prove that voluntary best practices work out over time. Supporting third-party audits, using renewable energy, and limiting hazardous waste set apart responsible suppliers from the rest.
Key Facts, Clear Choices
2-Amino-2',5-dichlorobenzophenone acts as a foundation, not a finish line. Its true value shows in how it fits into complex chemical crafting. Choosing the right partners, adopting better traceability, and following up with real care around health and environment draws the line between business as usual and lasting trust.
Understanding Its Structure
2-Amino-2',5-dichlorobenzophenone draws attention in the world of chemical research and manufacturing. The molecule, like a unique puzzle piece, comes together based on a clear-cut structure: two benzene rings linked by a carbonyl group, decorated with an amino group at the 2-position on one ring and chlorine atoms at the 2’ and 5 positions on the other. With the basic benzophenone skeleton (C13H9NOCl2), the actual arrangement determines how it behaves and interacts.
I’ve seen firsthand in the lab that once amines and chlorines both come into play on an aromatic framework, reactivity shifts, especially toward functional group modifications. The carbonyl bridge flanked by differently substituted rings gives chemists a way in for making designer molecules. These structural features make it more than a catalog entry; the set-up shapes applications in pharmaceuticals and advanced material synthesis.
Digging Into Structure: More Than Atoms on a Page
On paper, the core formula shows a carbonyl group snug between two benzene rings. Looking closer, the amino group at position 2 on one ring (the “left” side, if you imagine it that way) plays off the electron-withdrawing action of the chlorines at positions 2’ and 5’ on the other ring. This set of interactions sets the tone for how reactive this molecule stays under practical conditions.
In practical terms, those positions matter a lot. The 2-amino group tends to serve as a handle for building more complex derivatives, a starting point for new reactions. The chlorines on the other ring make that side less reactive, but more protected—handy for cases where you need stability under harsh conditions. Chlorine atoms often raise red flags in safety discussions, but here, properly handled, they support selectivity in synthesis and help create target drugs or agrochemicals with improved properties.
Why This Structure Is Important
Drug discovery hinges on combinations like this. With the right pattern of chlorine and amino groups, researchers can fine-tune properties like solubility and biological activity. Take it from someone who’s spent long hours comparing benzophenone-like candidates: small tweaks in position and attachment can mean the difference between a failed lead and a breakthrough compound.
Beyond pharma, the overall architecture helps in organic electronics, where robust and tunable aromatic systems serve as building blocks for light-absorbing or light-transporting devices. The electron withdrawing and donating groups (chlorines and amino) enable fine control over electron flow, vital for these applications. In my experience, a molecule like this, with its dual “personalities,” becomes a toolbox for pushing boundaries in both research and tech development.
Using This Structure Safely and Successfully
Safety with halogenated compounds means extra attention in labs and production plants. Those chlorine atoms may increase potency in a positive way, but they call for proper ventilation, disposal methods, and worker training to keep the environment and people safe. Policies based on up-to-date research and accident data help a lot; I’ve watched the impact after introducing stricter controls and rapid reporting—it turns a risky environment into one where people feel confident and protected.
The future benefits from open pathways between bench research, manufacturing, and regulatory groups—all focused on molecules like 2-Amino-2',5-dichlorobenzophenone. Clear, trustworthy communication ensures great ideas travel from experiment to shelf, helping people without sacrificing safety or quality. Staying grounded in the chemical structure keeps everyone moving forward, molecule by molecule.
How I Approach Unfamiliar Chemicals
Walking into a lab filled with unfamiliar reagents, I always check the labels, then think back to long afternoons spent in chemical safety training. When handling materials like 2-Amino-2',5-dichlorobenzophenone, being careful isn’t about following a strict rulebook; it’s about understanding why we should respect every step—because shortcuts in chemistry don’t forgive.
Wearing Protective Gear Is About More Than Just Compliance
I’ve worked next to folks who skip gloves or think a basic cotton mask does the trick. It doesn’t. Chemicals like this one can wreak havoc if they touch skin or get near the eyes and mouth. Splash-proof goggles and nitrile gloves help keep reactions where they belong: in the beaker. Long sleeves and lab coats might feel like overkill, but scars and rashes from errant drops will change your mind quickly.
Ventilation: Not Just a Box to Check Off
Breathing in powder or fumes, even from chemicals that seem mild, can lead to headaches or worse. Local exhaust hoods suck away vapors and keep the air clear. More than once, I’ve seen someone underestimate how fast a poorly ventilated bench gets uncomfortable. Open air doesn’t help—fume hoods make a difference by pulling harmful particles away before anyone breathes them in.
Knowing the Risks Pays Off
Reading the safety data sheet (SDS) sometimes feels dense, but those hazard statements and pictograms matter. With 2-Amino-2',5-dichlorobenzophenone, it’s worth checking for risks like toxicity if swallowed, skin irritation potential, or harmful dust. Once, a spilled gram seemed minor—until we realized how easily irritant dust spread into adjacent spaces.
Good Hygiene Can’t Be Skipped
After working with any chemical, nothing moves faster than folks heading to lunch without washing up. Washing hands with soap and water after handling is simple, but it makes a world of difference. Eating or drinking near the hood means you’re gambling with your health. Soap and a thorough rinse matter, not just a quick splash of water.
Safe Storage Saves Trouble Later
Materials like 2-Amino-2',5-dichlorobenzophenone need a cool, dry place—far from sunlight or heat, and away from incompatible solvents or acids. Once, a mislabeled bottle ended up next to a strong oxidizer and we spent a tense half-hour calling the safety office—plain shelving, clear labels, and routine checks save headaches and possible emergencies.
Prompt Cleanup and Spill Response—No Hesitation
Even a small spill can expand quickly, especially if powder scatters. Sweeping with care, using absorbent pads and keeping contaminated waste separate helps keep workspace and people safe. I’ve learned to circle spill kits before even opening a new jar. Reporting and documenting accidents, even if embarrassing, ensures that others know what happened and can prevent repeats.
Learning From Experience and Not Repeating Mistakes
Everyone slips up. Once, I tried to finish a synthesis in a hurry and ended up wiping crystals off my sleeve for days. Those mistakes stick in your mind. Shortcuts are tempting, especially on a busy day. Practicing habits like double-gloving, labeling everything, and recapping bottles every time pays off. You don’t need to be perfect, but staying honest about risks and respecting what’s on the bench protects both science and the people who do it.
Why Purity Matters on a Practical Level
When people talk chemistry, purity isn’t just a bragging point or a label on a spec sheet. Pure chemicals lead to clearer results and safer work. In synthesis labs, a high-purity product means fewer headaches at the bench. No one wants to track down whether a contaminant from their starting material torpedoed an experiment or if a little something extra snuck into the product—especially with 2-Amino-2',5-dichlorobenzophenone. This compound finds use not only in advanced organic synthesis but also in pharma and material science labs, where a tiny impurity can spark a chain of mistakes.
Industry Standards vs. Real-World Experience
Lab suppliers like Sigma-Aldrich or TCI list their high-purity chemicals at 97% or higher, measured by HPLC, GC, or NMR. These aren’t just arbitrary numbers—they reflect a balance between what's achievable and what's profitable. But even 97% isn’t always enough. In projects involving pharmaceuticals, that last three percent matters. Unwanted byproducts or residual solvents can cause everything from regulatory nightmares to failed reactions down the line. Over the years, I've seen projects stall because the supposed “trace” impurity in a crucial ketone like 2-Amino-2',5-dichlorobenzophenone triggered a side reaction.
Testing Isn’t Optional
Don’t assume the vendor’s paperwork tells the full story. Analytical testing in your own lab is crucial. A clean HPLC trace or a tidy NMR doesn’t just satisfy the quality control team—it brings confidence to the bench chemist. Instrument access costs time and money, but skipping that step risks the entire synthesis. More than once, I’ve loaded up a column for a quick check, caught a misidentified contaminant, and saved weeks of wasted work. This kind of diligence forms the backbone of scientific results that actually hold up.
More Than Just a Number
People outside research circles sometimes think product purity is just about prestige — but real consequences lurk if purity checks go slack. For example, contaminants in this compound can create signals in key steps or final products, making it hard to interpret results. Sometimes reactions fail, but the cause isn’t the chemistry—it’s hidden somewhere in a 2% impurity. So it makes sense to treat purity as a real world safety net.
Raising the Standard: Solutions and Habits
Improving outcomes relies on more than just trusting a vendor’s specs. Establishing in-house verification protocols helps catch surprises before they spiral. People often grumble about the time these checks steal from “real” science. In reality, thoughtful verification adds speed in the long run by cutting unplanned reruns and troubleshooting. Developing good supplier relationships helps too. If you notice batch variability or suspect a quality dip, speak up—responsible vendors will investigate and often improve process controls.
Some labs seek out custom purification or source their own starting materials if the stakes are high. Costs run higher, but knowing the purity down to a fraction of a percent often pays for itself. For 2-Amino-2',5-dichlorobenzophenone, I’ve found that using freshly purified material often solves tricky issues, especially when synthesizing sensitive downstream compounds.
Final Thoughts
In science, trust builds from evidence. You get that by running proper tests and maintaining standards, not by taking purity claims at face value. Years of working with organic syntheses taught me that taking shortcuts on purity rarely pays off. A little effort up front—through reliable suppliers, thorough analysis, and open channels for feedback—brings cleaner results and fewer headaches, every time.
Understanding the Risks Around This Chemical
Handling chemicals like 2-Amino-2',5-dichlorobenzophenone demands more effort than a quick glance at the label. This compound catches attention with its white to off-white crystalline form, and its presence in research and industry brings unique hazards. Most people aren’t going to see or handle this molecule at home, but in labs, proper storage keeps people safe and the substance effective.
People’s Health Comes First
Direct skin or eye contact with this chemical can cause irritation. Inhaling dust or vapors shouldn’t be taken lightly. As someone who’s spent time around chemical stocks, I’ve learned you can’t just toss compounds onto a shelf. Bad storage often leads to accidental exposure, which can mean anything from a rash to a bigger health scare.
Think About Where and How You Store It
2-Amino-2',5-dichlorobenzophenone responds badly to heat, moisture, and sunlight. A dry, cool, well-ventilated place works best. In my own lab experience, chemicals kept away from windows and radiators experience far fewer problems—clumping, odd odors, or even mysterious color changes. Temperature swings also form condensation inside containers, and moisture invites clumping or starts slow breakdown reactions.
Choose the Right Packaging
Many chemicals, including this one, do poorly in containers exposed to air for long. Sealing the container tight after every use is non-negotiable. Use containers with strong lids and labels that stand up to chemicals and wipe-downs. I once saw a jar leak just because its old label tape let moisture in around the edge. After that, we checked all our chemicals for seal integrity and swapped out tired containers without waiting another day.
Avoid Mix-Ups and Accidents
Some people stash chemicals alphabetically or by use, but I’ve found grouping them by hazard works out better. Acids, bases, oxidizers, and flammables shouldn’t even be near each other. 2-Amino-2',5-dichlorobenzophenone isn’t explosive, but it doesn’t play well next to strong oxidizers. Storing chemicals with their safety data sheets close by helps everyone—especially new team members—avoid mistakes and respond fast if something spills.
Monitoring Your Inventory Matters
Expired and degraded chemicals go from asset to problem quickly. A good checklist for regular inventory checks saves headaches. In my earlier days, nobody tracked open dates. More than once, we wondered why a reaction flopped, only to discover the reagent had aged out. Writing the date a bottle is opened and reviewing stocks every couple of months keeps things in check.
Training Beats Guesswork
Trusting workers to “just know” what to do doesn’t cut it. Regular training on storage protocols, emergency procedures, and safe handling pays off. Answering questions about these chemicals before an emergency turns staff from bystanders into a team that knows how to act. Feedback from coworkers often brings new, safer ideas to the table, too.
Looking Beyond Compliance
Regulations shape chemical storage, but what sticks with me is the culture around safety. Storing 2-Amino-2',5-dichlorobenzophenone safely protects both health and data. Clear systems, real-world experience, and teamwork bridge the gap between rulebooks and daily habits, keeping workplaces productive and people confident in their safety.