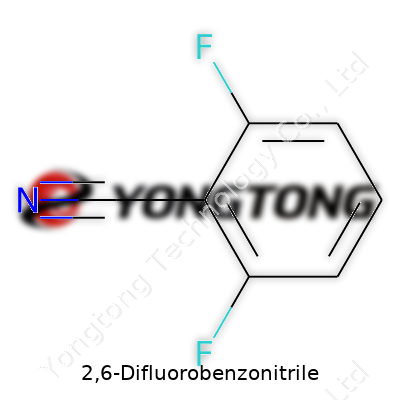
2,6-Difluorobenzonitrile: A Closer Look at a Subtle Powerhouse in Chemistry
Tracing Historical Paths
Chemists have always relied on the steady presence of aromatic nitriles to drive the fields of materials science, pharmaceuticals, and agrochemical development. 2,6-Difluorobenzonitrile, known by the shorthand DFB, first entered the scene during the boom of halogenation techniques in the twentieth century. Technological advances and a hunger for new molecular scaffolds naturally encouraged exploration into fluorinated aromatics. DFB emerged out of research focused on harnessing the unique properties of fluorine atoms within organic building blocks, providing chemists a sturdy platform to create advanced molecules for drugs and crop-protection agents. The molecule’s journey has paralleled larger changes in synthetic chemistry: a pivot from simple transformations toward more controlled, precise manipulations of molecular architecture. In industry and research, DFB has quietly worked its way into applications that demand strict consistency and predictable behavior, demonstrating that incremental tweaks in molecular structure can ripple through to sizable impacts in final products.
Product Overview
2,6-Difluorobenzonitrile presents itself as a pale crystalline solid. Not much to look at, but beneath its plain surface, it packs plenty of chemical potential. Its role isn’t always front-page material, yet it works deep within synthetic routes for dyes, pharmaceuticals, and specialty polymers. What hooks professionals is its dual nature: the benzene ring’s stability and the nitrile group’s chemical reactivity, flanked by two stubbornly electronegative fluorine atoms. That setup opens doors for tight control in reactions, whether the aim is to tweak biological activity in drug synthesis or generate tough, versatile resins with precise thermal properties. The confidence that DFB brings to a process rests on decades of reliability and reproducibility. Whether in a pilot plant scaling up an API, or a small lab searching for the next blockbuster crop-protection agent, DFB delivers time and again.
Physical & Chemical Properties
DFB doesn’t waste much time with theatrics — it’s solid at room temperature, off-white in color, and sports a faint, almost sweet odor that betrays its organic backbone. Its melting point sits around 41-45 °C, but that’s just the tip of the iceberg. Its boiling point nudges past 200°C, demonstrating enough thermal resilience to make it a capable intermediate for high-temperature reactions. Water doesn’t mix well with DFB; it’s hydrophobic, preferring organic solvents such as acetonitrile, toluene, or dichloromethane. Log P values hover in the predicted range for fluorinated aromatics, usually close to 2, which spells out modest lipophilicity and gives a sense of how it navigates biological membranes during development of pharmaceuticals or pest-control agents. Its electronic nature draws from the combined influence of the electron-withdrawing cyano group and the double fluorination, resulting in specific reactivity patterns, especially in nucleophilic aromatic substitution.
Technical Specifications & Labeling
Quality standards for 2,6-difluorobenzonitrile carry significant weight. Purity matters, usually 98% or above for anything destined for medical or electronic use. Color, melting point, and residual solvent content round out the typical technical profile, along with IR and NMR fingerprints for batch identification. Regulatory frameworks demand full traceability. Proper labeling includes CAS number (1897-52-5), molecular formula (C7H3F2N), and data sheets citing hazard statements under GHS regulations, not to mention supplier batch numbers for downstream recalls or validations. Every shipment comes with its own paperwork stack — safety data sheets, COAs, and handling instructions. This paperwork may feel tedious, yet it protects everyone up and down the chain, from the chemical manufacturer right through to the research technician weighing out the powder in a university lab.
Preparation Methods
For years, the best syntheses of DFB leaned on halide exchange chemistry. A typical sequence starts from 2,6-difluorotoluene, which undergoes controlled oxidation to its corresponding benzoic acid followed by a dehydration to install the critical nitrile group. Commercial routes tend toward process safety and scalability, using cheap oxidizers and minimal toxic byproducts. Newer approaches have focused on greener methodology: phase-transfer catalysis, continuous-flow platforms, and solvent-recycling cycles have all helped reduce emissions and cost, echoing broader trends in sustainable manufacturing. The push for efficiency and environmental stewardship hasn’t dulled production, instead inviting creative minds to chart routes that squeeze waste, limit use of rare metals, and recycle side streams wherever possible. Young chemists get their hands on both the legacy textbook preparations and recent publications, finding that smart process engineering matters as much as stoichiometry.
Chemical Reactions & Modifications
The presence of two fluorines in the ortho positions gives DFB an unusual reactivity profile, especially toward nucleophilic aromatic substitution. This uniquely positions it for use in making a host of polyfunctional molecules — from ligands for advanced catalysis to intermediates for pharmaceuticals. Removal or exchange of the fluorines allows for the installation of other functional groups without disturbing the nitrile – a trick that leads to libraries of derivatives for screening in medicinal chemistry. The nitrile opens further synthetic pathways, inviting conversions into amides, amidines, and carboxylic acids. Electrophilic substitution can be tricky because of the electron-withdrawing crowd; that said, selective halogenation or metalation remains possible under the right conditions. In practice, DFB acts as a reliable scaffold, one that lets chemists play with the fine balance between electronic and steric effects in molecular design.
Synonyms & Product Names
Scanning catalogs and chemical patents, one quickly learns 2,6-difluorobenzonitrile is a molecule with many aliases. It’s often listed as 2,6-DFBN, α,α-difluorobenzonitrile, or by less formal descriptors like ortho,ortho-difluorobenzonitrile. Whether in English, German, or Japanese chemical catalogs, the molecular structure trumps the name, but clarity on labels is non-negotiable when importing or exporting across borders.
Safety & Operational Standards
Handling DFB demands caution and respect, just like with any organofluorine compound. Eye protection and gloves aren’t negotiable in the lab. Prolonged inhalation or skin contact brings risk ranging from mild irritation up to more serious respiratory symptoms, dictated both by concentration and exposure time. Facilities rely on local exhaust ventilation, dedicated chemical fume hoods, and spill containment plans. Storage must keep the compound dry, away from oxidizing agents, strong bases, or acids that could trigger decomposition or uncontrolled release of toxic byproducts. Emergency protocols cover accidental releases or exposure, with clear instructions on decontamination and medical attention. Practicing chemists keep a watchful eye toward emerging safety bulletins or updated recommendations from regulatory agencies, as ongoing research sometimes uncovers new risks or changes old assumptions.
Application Area
DFB serves as a workhorse in several technical fields. Medicinal chemists often use it to build candidate drugs, harnessing its reactivity to bolt on new chemical features that modulate biological activity. In crop-protection science, it links up with other fluorinated components to tune the environmental lifetime of new herbicides or insecticides. Material chemists chase its unique blend of attributes for crafting performance polymers, LCD intermediates, or specialty dyes. Its utility doesn’t always show up in the headlines but yields tangible results — a coating that resists weathering a bit better, a targeted cancer therapy molecule, an OLED color layer engineered for longevity. Each sector brings its own demands, but DFB meets them by standing firm and predictable in the face of tough technical challenges.
Research, Development, and Toxicity Research
Research on 2,6-difluorobenzonitrile continues to uncover new technical uses and potential health impacts. Structured studies examine its behavior in biological systems, including mutagenicity, biodegradation, and chronic exposure risks. So far, acute oral and dermal toxicity seem low compared to many other aromatic nitriles, but ongoing work watches for metabolites or byproducts that could complicate the picture. Emerging data from academic and commercial labs inform updates on occupational exposure limits and waste management regulations. In a laboratory or manufacturing setting, training and vigilance reduce the risk of runaway reactions, fires, or toxic emissions. Teams looking to launch new products based on DFB derivatives frequently engage in extensive preclinical testing to confirm safety, environmental fate, and compliance with evolving chemical safety laws around the globe. As DFB continues to climb up the value chain, balancing technical payoff with responsible stewardship draws as much attention as yield or purity.
Future Prospects
Looking ahead, the future of 2,6-difluorobenzonitrile seems secure in a world that increasingly values specialization, reliability, and targeted molecular properties. The continued push for greener chemistry promises to refine its manufacturing and downstream transformations further, dialing back emissions and improving waste recovery. Developments in pharmaceutical research suggest that fluorinated aromatics, including DFB, will anchor new generations of tumor-targeting drugs and metabolic modulators. Digital technologies — machine learning, cheminformatics — fuel an acceleration in the discovery of new derivatives and synthesis pathways, offering researchers a better toolkit for navigating chemical space. Healthy debate remains about its longer-term environmental profile, especially regarding persistence and breakdown products in soil and water. Still, close collaboration among academia, industry, and regulators will keep DFB and its derivatives on a responsible course. Those who work with it every day know that behind each batch rests a network of technical choices, scientific rigor, and a stubborn attention to detail that transforms an unassuming molecule into an engine of progress for chemistry and allied sciences.
Beneath the Surface of the Formula
Chemistry gets a reputation for sticking to complicated-looking letters and numbers, yet diving into those formulas brings a lot of sense to the way things work—especially with compounds like 2,6-difluorobenzonitrile. For many people who’ve worked in a laboratory or just navigated modern living, figuring out what a chemical formula reveals can put real-world power in your hands. Here, the focus rests on 2,6-difluorobenzonitrile and its formula: C7H3F2N.
Chemical Details and Real-World Value
Those seven carbons, three hydrogens, two fluorines, and a single nitrogen offer more than just a name. Placing hydrogens and fluorines around a benzene ring isn’t just some dry academic task. The interactions in these molecules spark long research hours worldwide, forming the backbone for smarter materials and sharper pharmaceuticals. In the industry, I’ve seen this substance pop up in synthesis work where precision makes the difference between a failed reaction and a product worth patenting. The introduction of fluorine changes properties: it resists breakdown, stays stable under wild conditions, and brings a toughness that’s hard to match.
With my own hands on benchtops, the value of memorizing and understanding formulas like C7H3F2N goes beyond classroom tests. It boils down to safety too. Even small shifts in a formula (a moved fluorine atom, swapped for another halogen) can mean you face a different hazard. I’ve watched colleagues triple-check bottle labels, because one digit off in a formula messes up reaction planning. You run into lost resources and safety risks much faster than you’d expect.
Why Knowing the Formula Matters
2,6-difluorobenzonitrile doesn’t end up in your kitchen, but it often leaves fingerprints on things you use every day. Electronics benefit from fluorinated aromatics, and some advanced medicines trace roots to compounds in this family. The structure tells chemists a lot. I learned early that a benzonitrile component brings a high reactivity in synthesis, and fluorines at position 2 and 6 slow down unwanted side reactions. This control boosts yields and reduces toxic waste—a big deal if you tally up what a factory can dump by accident.
Students and professionals tripping over the formula in textbooks or safety data sheets aren’t just hunting trivia. The right formula keeps the entire chain of operations flowing smoother. One misstep, and you get ruined products, wasted time, and potentially dangerous byproducts.
Improving Outcomes: Sharing Knowledge, Ensuring Responsibility
Wider education about small but potent molecules pays off. Open discussions among chemists, safety experts, and industry leaders can put everyone on the same page, which means fewer accidents and more reliable finished goods. In my experience, teams who could both rattle off the right formula and explain what each atom means tended to solve problems faster and keep workplaces safer. Collaborating with researchers and supporting robust chemical education whittles down mistakes. When everyone on the team, from new hires to management, recognizes the difference a formula brings, process quality naturally steps up.
Why This Compound Matters
Everyday items, from the aspirin you take for a headache to the plastics that store your leftovers, depend on certain raw molecules to become reality. 2,6-Difluorobenzonitrile is one of those molecules that doesn’t show up on most people’s radar but quietly gets the job done behind the scenes. I started looking into this stuff thinking it might just be a boring chemical, but I quickly learned that it plays a pretty clever role in several high-impact fields.
Pharmaceuticals: Quiet Force in Drug Development
Drug companies often look for molecules that help make more complex medicines. 2,6-Difluorobenzonitrile works really well here. It forms the backbone for products like anti-inflammatory drugs and treatments for neurological disorders. The difluoro part changes the chemical behavior, making it easier to control a drug’s metabolism in the body. Researchers find this helpful when seeking improved selectivity—getting more targeted action with fewer side effects. It is a great example of how a modest compound, in the right spot, can shift the entire effectiveness of a medicine.
Pesticides: Essential for Modern Agriculture
Ask anyone who works on a farm, and you’ll hear stories about how pest control makes all the difference in keeping food affordable and healthy. Chemists take 2,6-Difluorobenzonitrile and use it to synthesize active ingredients in a range of pesticides. The fluorine atoms change the way insects and weeds interact with these chemicals, creating more robust crop protection formulas. Without this compound, large-scale farming could struggle more with resistant pests.
Material Science: Engineering New Possibilities
Polymers and advanced plastics often require special building blocks to gain new features, such as heat or chemical resistance. In the lab, teams have relied on 2,6-Difluorobenzonitrile to put these benefits into practice. Its structure lets scientists add fluorine—which is tough and stable—alongside a nitrile group that helps with strong linkages. You can find echoes of this compound in everyday items like coatings, adhesives, and circuit boards, all of which run better because of it.
Challenges With Handling
Working with any chemical brings safety concerns, and 2,6-Difluorobenzonitrile has its own. Direct skin contact or inhalation could be harmful. The factories and research centers using this compound rely on controlled environments, personal protective gear, and strict storage protocols. It’s a good example of why chemical labs spend as much time on training as they do on experiments.
Moving Forward: Responsible Use
Like many industrial and pharmaceutical chemicals, responsible sourcing and safe handling go hand-in-hand with wider benefits. Companies that use 2,6-Difluorobenzonitrile focus more on transparent supply chains and keeping waste to a minimum. Green chemistry methods now let some manufacturers recover solvents and limit byproducts, helping the process stay both safe and sustainable. Change in this area doesn’t always make headlines, but it ripples out to make a real difference in daily life, from the medicines we count on to the food we put on the table.
Why Attention To Chemical Safety Is Essential
Working with chemicals like 2,6-Difluorobenzonitrile demands respect for the dangers they bring. This compound finds its place in labs that handle pharmaceutical research, fine chemicals, and dye intermediates. From what I’ve seen, one careless moment with a bottle of hazardous powder or liquid can create a mess that takes days or even weeks to fix—sometimes at the cost of someone’s well-being. The cyanide group and the fluorinated ring signal clear danger: this compound will attack the body’s systems if given a chance, either through the lungs or skin.
Proper Gear Means Fewer Regrets
Before even lifting a single vial, lab staff should have good personal protective gear. This means reliable chemical-resistant gloves—nitrile or neoprene, not the blue latex ones often used with less risky solutions. Goggles that hug the face block splashes far better than reading glasses, which betray when a liquid jumps up. Lab coats made of heavy cotton or synthetic blends shield the arms, and closed shoes cover more than toes. I've watched friends regret working in shorts during summer—chemical burns pay no attention to weather.
Good Air Is Good Health
2,6-Difluorobenzonitrile’s vapors don’t just smell sharp; they threaten with toxicity to the nose and lungs. Fume hoods offer more than just peace of mind, filtering out fumes before anyone breathes them in. Cracked open windows or simple fans can’t do this job. In any lab running dozens of experiments at once, I’ve noticed the difference a strong exhaust system makes for the whole team's comfort and long-term health.
Handling And Storage: Details Prevent Big Mistakes
Keeping this chemical tightly sealed in bottles meant for volatile poisons helps keep accidental spills rare. Labels need to stand out, listing both its real name and a skull symbol. I always double-check bottles after seeing incidents where someone grabbed a clear jar in a rush, expecting one compound, only to pour another. Store it away from acids and strong bases, since an unplanned reaction can quickly turn dramatic. Fireproof cabinets add another layer of confidence, because even small amounts of this type of reagent feed dangerous blazes.
What To Do After A Spill Or Exposure
Spills send both nerves and protocols racing. If some drink up your sleeve or land on your glove, pull off anything that’s wet and wash up with running water—ten solid minutes at least. Report even small exposures, since cumulative poison can sneak up within hours. The safety shower and eyewash station prove their worth when someone’s vision or skin saves seconds. In my career, I have never forgotten the panic on a colleague’s face when splashed with something toxic—a well-practiced routine calmed everyone and helped the injured person recover.
Training Builds Confidence, Not Just Compliance
No amount of fancy equipment saves the day when people just guess what to do. Regular drills, safety briefings, and hands-on training in that specific lab—with the real gear and real protocols—mean fewer accidents. New team members pick up habits from old hands: double-bagging waste, reading every label three times, pausing before mixing anything. Safety culture doesn’t come in a binder; it grows where everyone buys in.
The Bottom Line
Handling compounds like 2,6-Difluorobenzonitrile takes more than a checklist. It takes vigilance, honesty about near-misses, and a willingness to ask for help with unfamiliar steps. Real-world safety grows out of habits and teamwork, not theory.
Getting to Know 2,6-Difluorobenzonitrile
2,6-Difluorobenzonitrile carries a simple formula, C7H3F2N, and a complexity that matters in the lab and industry. For many years, chemists have counted on accurate physical data to map out safe handling and efficient reactions. The precise melting and boiling points of a compound like this aren’t just numbers—they dictate storage, transportation, and use. The melting point hovers around 35°C (95°F), while the boiling point comes in at approximately 210–211°C (410–412°F). These aren’t figures conjured up in passing. They come from painstaking measurement, peer-reviewed sources, and industrial safety data sheets.
Why Physical Properties Matter in Everyday Science
Anyone who works with chemicals, whether in pharmaceuticals, agriculture, or advanced materials, knows that these temperature limits play a direct role in shaping decisions. In the solid state below 35°C, this molecule remains stable and packs well. Once the temperature climbs above that mark, the substance transitions into a liquid. Lab technicians can pour it, mix it, or dissolve it without fuss. Solid-to-liquid change is practical knowledge, not trivia—mishandling volatile solids and liquids can cost time, money, and sometimes more.
The boiling point, over 210°C, shows fewer surprises on the workbench but raises big questions for engineers and safety officers. High temperatures set a clear danger zone. Heating above the boiling mark triggers vapor release, which demands good ventilation, correct containment, and sometimes special protective gear. In older chemistry labs, open flames or poorly controlled heaters brought out the worst in volatile organics, leading to spills, clouds of fumes, or intense headaches by lunchtime. Decent knowledge—like not boiling a material with a closed lid—saves both health and budgets from avoidable risks.
Connecting the Dots: Safety, Efficiency, and Progress
The importance goes beyond chemist curiosity. Take drug discovery, where molecules like 2,6-difluorobenzonitrile help build complex pharmaceuticals. Knowing exactly when it melts or boils directs every step, from weighing out samples to scaling up kilograms in a pilot plant. A chemist who ignores those precise marks struggles with product quality or safety shut-downs mid-synthesis—that means lost time, lost money, and sidetracked innovation.
It’s tempting to skim the spec sheet and trust someone else’s numbers, but primary safety incidents often come from complacency. In one of my own early research stints, a rushed solvent swap wrecked an entire run. Vapors from a poorly chosen heating step set off alarms and ruined hours of preparation. That memory lingers and remains a daily reminder: know the temperatures, respect the risks, and keep communication clear among everyone handling the material.
Building on Reliable Data
The chemical world relies on more than experience and intuition. Reliable, up-to-date reference data comes from groups like the National Institute of Standards and Technology and major chemical suppliers. Industry compliance teams and research leaders expect every chemist to verify numbers rather than rely on photocopies or online scraps. Small missteps scale quickly in industry—wrong storage temperatures, for example, can force recalls or shut down a plant line for days.
So, if chemical safety and progress matter, it starts with tools as humble as a thermometer and as crucial as a verified spec sheet. Seemingly simple data points anchor real-world safety, keep projects running, and breathe confidence into teams building our next round of medicines, polymers, or specialized materials.
Letting Chemicals Teach Us Respect
Sitting in a lab, you learn pretty quickly that every bottle and drum carries risk. Take 2,6-Difluorobenzonitrile. Behind that mouthful, you’ve got a chemical that acts as a useful building block in pharmaceuticals and agrochemicals. At the same time, one wrong move can ruin your day—or your week. It stands out because the molecules pack cyanide alongside fluorine, so this isn’t the type of stuff you keep on a forgotten shelf. The most striking thing about storing it is how a little extra care gives peace of mind—and avoids major headaches.
Why Temperature Control Isn’t Just a Technicality
I remember walking into one lab after the air conditioning failed. It’s not a simple problem of personal comfort. Slight temperature changes can change how a compound behaves or breaks down. Chemicals like 2,6-Difluorobenzonitrile ought to stay cool, ideally below 25°C (77°F), away from heat and direct sunlight. Warmth speeds up decomposition or lets vapors escape, and with cyanide in the mix, you want to prevent both. Cold and dry storage cuts the odds of leaks or pressure build-up, keeping both people and product safe.
Sealed Containers Show Respect for Everyone
Glass bottles or HDPE jars with tight-fitting lids aren’t just about technical specs. Scratched, weak or ill-fitting containers have caused more messes than I care to count. With 2,6-Difluorobenzonitrile, vapors or spills could trigger serious health issues quickly. Corrosion-resistant, chemical-compatible containers—labeled and dated—make sure nobody guesses what’s inside. A casual label or a worn-out cap doesn’t cut it. Double containment in trays gives one more backup against breaches, and it sends a message: we value each other’s safety more than cutting corners.
Avoiding Water, Humidity, and Bad Surprises
Moisture changes the game. Even a splash from a leaky pipe or a damp shelf raises the chance of breakdown or reaction. Keeping 2,6-Difluorobenzonitrile in a desiccator with a drying agent or in a moisture-controlled storage room shrinks that risk. Humidity in labs eats away at confidence—people start second-guessing the safety of every bottle. Investing in dry storage means never wondering if the next bottle has become a hazard overnight.
Staff Training and Clarity
Years in the lab taught me that clear communication saves lives. Safety data sheets shouldn’t disappear in a filing cabinet. Regular training, review of chemical hazards, and emergency protocols turn a risky environment into a safer workplace. It helps when everyone, from the most junior assistant to the principal investigator, knows the drill—eye protection, gloves, ventilation, spill kits, and emergency contacts on hand. Honest discussions about near-misses reach further than posters on the wall.
Inventory Checks and Small Quantities Win Out
Large chemicals stockpiles tempt fate. With 2,6-Difluorobenzonitrile, I’ve seen teams work smarter by keeping only what they expect to use in the short term. Old, forgotten chemicals lose their labels, degrade, or get misplaced. A lean inventory—reviewed regularly—reduces disasters. Quick disposal of expired or unused reagents stops them from becoming tomorrow’s emergency.
Real Accountability
Behind every storage policy, there’s a person making choices. Sometimes, it takes courage to slow down, question shortcuts, or push for safer practices. Trust comes from everyone buying into these steps—never just out of fear, but out of self-respect and care for coworkers. In practice, careful storage of 2,6-Difluorobenzonitrile means building habits that last and having the nerve to enforce them every time.