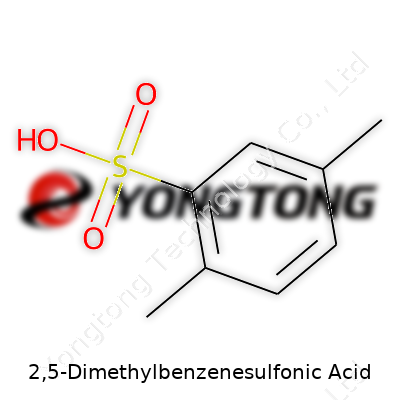
2,5-Dimethylbenzenesulfonic Acid: A Commentary
Historical Development
Looking at the path 2,5-dimethylbenzenesulfonic acid carved out in the world of chemistry brings to mind industrial progress through the late nineteenth and early twentieth centuries. Chemists and manufacturers searched for ways to tap into the versatility of aromatic compounds, eyeing alkylated benzenes as building blocks. The introduction of sulfonic groups marked a key advance, making these compounds far more useful in water-based systems and synthetic applications. As early synthetic dyes and detergents took off, demand for functionalized aromatics such as 2,5-dimethylbenzenesulfonic acid surged. Lab notebooks from those early years tell stories of creativity amid trial and error: workers armed with glassware, rudimentary analytical tools, and plenty of patience. Progress wasn’t just the result of scientific curiosity but also a reflection of growing industrial needs—textiles, cleaning products, water treatment, and specialty chemicals all pushed for new sulfonic acid derivatives. By the late twentieth century, commercial production processes refined yields and purity to support large-scale operations, setting standards that scientists and manufacturers still echo today.
Product Overview
2,5-Dimethylbenzenesulfonic acid stands out as an important arylsulfonic acid, known for its affinity to both organic solvents and water systems, a trait anyone running a lab finds it tough to replace. The compound shows up as a white to off-white solid, with clear, pungent notes demanding careful handling. Chemists across research, manufacturing, and quality control recognize its reliability for sulfonation reactions. Companies bundle it under several product names to meet different target audiences—“xylenesulfonic acid” as shorthand, and sometimes “2,5-DMBS acid” in technical datasheets. Its role reaches beyond simple reactivity: it shapes the start of synthesis for dyes, pharmaceuticals, resins, and specialty polymers. Even a seasoned operator develops a healthy appreciation for the acid’s ability to introduce consistent sulfonic groups without setting off a host of side reactions.
Physical & Chemical Properties
Handling 2,5-dimethylbenzenesulfonic acid day in, day out, you notice certain features fast: moderate melting point, usually around 120–125°C, it dissolves with ease in polar solvents, especially water and lower alcohols. Its solubility in nonpolar solvents dips, an advantage for separation techniques and purification. Its acidic strength hovers in line with related sulfonic acids, lending it enough power to catalyze reactions or adjust pH in robust settings. The two methyl groups at the 2 and 5 positions confer steric protection, slowing unwanted oxidation but still giving the aromatic core room to accept further substitutions under suitable conditions. During handling, its strong hydrophilicity draws moisture from air, so open containers sit capped tight in storerooms. Odor and hygroscopic behavior stick in your mind if you’ve ever weighed or sampled it for analytical work.
Technical Specifications & Labeling
Industrial and research settings demand specific details. Each lot of 2,5-dimethylbenzenesulfonic acid bears a certificate with CAS number, purity levels often exceeding 98%, moisture content, color grade, and residual xylene content. Suppliers must mark packaging with hazard identification codes, United Nations numbers for shipping, gross and net weight data, and the batch for full traceability in the wake of regulatory pressure. Simple mislabeling in the lab puts both people and research budgets at risk, so rigorous double-checking shapes the daily routine. The reach of GHS labeling now stretches to even the smallest containers, ensuring everyone understands the risks and properties before the jar ever hits the benchtop.
Preparation Method
Chemists got creative in synthesizing 2,5-dimethylbenzenesulfonic acid, turning to direct sulfonation of xylene precursors. Today’s synthesis typically starts with 2,5-dimethylbenzene, often known as meta-xylene, reacting with strong sulfonating agents like fuming sulfuric acid or oleum under controlled temperatures. The process relies on close management of heat and reaction time to avoid polysulfonation or breakdown of the aromatic ring, challenges frontline operators learn to finesse through practice. Crystallization steps and careful pH adjustments follow, yielding product as a pure acid or ready-to-use sodium or potassium salt, depending on downstream needs. In large reactors, automation and sensor feedback have taken over, but the underlying principles—thermodynamics, mass balance, and risk management—still draw from the hands-on lessons of the past.
Chemical Reactions & Modifications
2,5-Dimethylbenzenesulfonic acid acts as both a workhorse and a precision tool. In one sense, experienced chemists rely on its steady sulfonation properties for introducing sulfonic groups on aromatic rings, supporting functionalization in dye and resin production. In another, its ortho- and para-directing effects control the position of further substitutions, an advantage for tailoring molecular architectures. The acid group enables formation of metal salts, making it accessible for catalysis and ion-exchange applications. Researchers often modify the methyl positions to create tailored analogs, each opening new lanes in pharmaceutical and intermediate synthesis. Hydrolysis, coupling, and halogenation reactions occur readily under laboratory conditions, making this acid part of many catalog numbers for custom chemical suppliers. While a straightforward molecule, the range of derivative reactions brings serious versatility to synthetic planning.
Synonyms & Product Names
Anyone paging through supplier catalogs or academic papers encounters the same handful of names for 2,5-dimethylbenzenesulfonic acid. Besides the formal IUPAC name, you spot “2,5-xylenesulfonic acid” used interchangeably. Chemists drop the “acid” and substitute “sulfonate” when referring to its salts, though the core molecule remains the same. Product labels often shorten to “DMBSA” for inventory simplicity. This overlapping nomenclature calls for vigilance: confusion about structure or intended use leads to mistakes, whether at the order desk or in the lab. For routine users, the rhythm of double-checking every identifier, every synonym, becomes a second nature.
Safety & Operational Standards
With chemistry comes risk, and 2,5-dimethylbenzenesulfonic acid asks for care on the bench. Even seasoned techs don gloves, goggles, and face shields after seeing firsthand the irritation this acid can cause upon skin or eye contact. Spills near open containers lead to burns or lingering odors that fill the room. Strict air handling, spill response training, and documented risk assessments dictate everyday practice. Storage stays below 30°C, away from oxidizers and bases. Some facilities go further, tracking every gram of acid from delivery through disposal to tighten safety compliance with OSHA, EPA, and international shipping laws. Emergency eye washes, maintained spill kits, and regular refresher courses no longer feel optional once you’ve witnessed an incident or fielded a late-night call about a chemical exposure.
Application Area
The reach of 2,5-dimethylbenzenesulfonic acid extends from dye manufacturing plants to specialty polymer labs and water softening operations. As a sulfonating agent, it sets the stage for vivid, colorfast dye production, linking directly to progress in textiles and printing. In polymer science, adding sulfonic groups improves water compatibility, driving the performance of ion-exchange resins, fuel cell membranes, and specialty plastics. Block copolymer synthesis depends on such functional acids for predictable architecture and charge control. Oilfield services, detergents, and wastewater treatment teams all call on this acid in blends to tackle solubilization and scale challenges. My own brush with resin formulation underscored the value of consistency batch-to-batch; stray trace amounts of impurities skewed everything from color to performance, reminding us of the compound’s central role in high-spec applications.
Research & Development
Every time the synthesis of a new surfactant or specialty polymer hits the lab planning stage, 2,5-dimethylbenzenesulfonic acid turns up in formulation meetings as a go-to functional starting material. Researchers treat it as a platform for sulfonic group introduction and as a precursor to more complex sulfur-containing molecules. Innovation in “green” chemistry circles around milder sulfonation methods or biodegradable derivatives, all sparked by the core structure’s reliability. Pilot plants and analytical labs devote resources to new analytical standards that guarantee trace contamination levels. Postdoctoral teams and seasoned process engineers debate new salt forms or composite resins, sometimes returning to data drawn from the acid’s earliest handling protocols. Each development effort, from high-throughput screening to gram-scale proof-of-concept, leans heavily on access to high-purity material and reliable supply chain sources.
Toxicity Research
Facing safety audits, I’ve read plenty of toxicology reports on aromatic sulfonic acids. For 2,5-dimethylbenzenesulfonic acid, current research shows low acute toxicity in mammals. The acid’s corrosive properties toward the skin and eyes prompt routine medical checks for the lab crew, especially after accidental exposure cases that require longer follow-up. Long-term effects remain less studied, but most safety data suggests low environmental persistence thanks to its relatively good biodegradability compared to unsulfonated aromatics. Regulatory agencies list the acid under controlled substances needing appropriate handling and disposal, particularly in bulk chemical plants. Following best practices limits risk, and so far the published data supports its continued use in regular research, manufacturing, and development contexts, provided teams stick to guidelines.
Future Prospects
Looking ahead, 2,5-dimethylbenzenesulfonic acid seems set for a further run in synthetic chemistry and industrial materials. The push for sustainable chemical processes brings a chance to revisit classic sulfonation methods, seeking cleaner catalysts and energy savings, both for safety and emissions. New functional materials for batteries, membranes, and advanced resins lean on the acid’s flexible reactivity. The ongoing trend toward personalized polymers and Arylsulfonic-based pharmaceuticals will likely spark yet more adaptation, demanding tighter controls on trace contaminants and innovative modifications. Research continues to refine the environmental footprint, balancing biodegradability with long-term stability in specialty products. From first-hand experience, keeping a finger on industry collaborations and regulatory updates pays off; the evolution of such cornerstone chemicals often brings new standards, requiring chemists and operators to pivot as expectations change in the decades ahead.
Chemical in the Details
2,5-Dimethylbenzenesulfonic acid tends to hide behind a complicated name, but it gets used in all sorts of everyday things. Chemistry classrooms might treat it as just another sulfonic acid, but in real life, this compound means practical outcomes. In the lab, folks know it as a strong acid. Outside, it shapes the products people trust—often without them realizing it.
Connections to Cleaners and Dyes
Most cleaning products you see these days rely on surfactants. These surfactants help grime lift off and wash away with water. Here’s where 2,5-dimethylbenzenesulfonic acid leaves a mark. Chemical plants turn this acid into salts and intermediates for a class of surfactants called alkylbenzenesulfonates. These surfactants power soaps, liquid detergents, and industrial cleaners. Anyone who’s cleaned a shirt or scrubbed a metal part has probably counted on these products, even if they never checked the ingredient list.
Manufacturers also lean on this compound for dyes. The acid’s strong sulfonic group makes colors more water-soluble. Textile mills want bright, long-lasting shades, so the chemical steps in as a builder for certain dyes. As a result, towels keep their color and blue jeans hold up in the wash. Otherwise, we’d all face fading fabrics and dull hues—something nobody wants.
Supporting Pharmaceuticals and Specialty Chemicals
Medicines people take can owe some properties to strong acids like this one. Pharmaceutical companies sometimes use 2,5-dimethylbenzenesulfonic acid to tweak the solubility or stability of drugs. In my grad school days, research groups often needed strong acids to produce solid forms of candidate medicines. If a new pill dissolves or absorbs better, patients see the benefit directly. That’s one of the unsung stories behind medicine development.
On top of this, the chemical connects to the world of catalysts. In organic synthesis, reactions need a nudge to move faster or more selectively—strong acids do the job. Whether a company needs to build a medical compound or a fragrance ingredient, this acid has shown up in the recipe book, making complicated chemistry possible at a larger scale. Anyone who’s worked on new reaction methods understands how crucial a reliable catalyst can be in hitting production targets and keeping costs in line.
Looking at Challenges—And Solutions
Strong acids come with baggage. Factories handling chemicals like 2,5-dimethylbenzenesulfonic acid must put strict safety routines in place. Skin contact burns, fumes can irritate, and if waste isn’t managed, downstream rivers suffer. We’ve all seen stories where chemicals leak, and nobody wants that nightmare. Engineering controls—proper ventilation, tight containers, and real-time monitors—keep accidents rare. Sites investing in equipment upgrades have a better shot at stopping spills and protecting workers.
On the environmental front, the story matters just as much. Modern wastewater plants break down these acids, mostly through advanced oxidation. Still, not every country runs the cleanest water treatment programs. It takes investment, regular monitoring, and public attention. Here, pressure from watchdog groups and transparent reporting drives better habits. That said, most users and producers in developed markets follow rules to the letter. Policymakers working with industry leaders can push safer substitutes and encourage greener methods where possible. If communities and regulators speak up for higher standards, it’s easier to balance chemical progress with safety and clean water.
Digging Into the Basics
Anyone who’s ever tried to understand what they’re working with in a lab knows formulas and numbers matter. If you’re handling 2,5-Dimethylbenzenesulfonic acid, you want facts plain and simple. Its chemical formula is C8H10O3S. Staring at that formula, it’s more than just letters and numbers. You see a benzene ring, which forms the chemical backbone many industries lean on, with methyl groups hanging off at the 2 and 5 spots, plus a sulfonic acid group.
Molecular weight gives chemists a reference for many daily decisions—calculations for reactions or just preparing the right amount for testing. For 2,5-Dimethylbenzenesulfonic acid, this sits at 186.23 g/mol. Each atom adds up: carbon brings 8 x 12, hydrogen at 10 x 1, sulfur adds its unique heft at 32, and three oxygens combine for another 48. Anyone used to weighing out grams for synthesis knows the importance of accuracy—one slip and experimental results slide off track.
Why These Details Grab Attention
The layout of this compound means more than most folks guess. Adding two methyl groups on the benzene core changes how the molecule interacts with just about everything—from solubility in water to its role as a building block in chemical manufacturing. Labs, especially those focused on dyes or surfactants, care deeply about this. I’ve seen how a small change boosts solubility, which shoots efficiency up and sometimes reduces waste. Less fuss means less clean-up and fewer headaches.
In industry settings, knowing what’s in the bottle isn’t just about being precise for the sake of it—costs build fast when mistakes creep in. A missed calculation on molecular weight can throw an entire batch of product out of specification. No one wants to explain why a shipment doesn’t measure up, especially with the financial stakes involved in chemicals like sulfonic acids.
Making Chemistry Safer and Smarter
Safety should never land on the back burner. Misidentification of chemicals turns dangerous before you know it, especially with sulfonic acids, which bring strong acidity and need proper handling. Getting the formula right helps confirm compatibility with containers, pipes, and lab gear. Too many times, skipped details lead to equipment wear or chemical burns, both of which could have been dodged.
Molecular weight isn’t just academic—it ties right into how we measure toxicity, environmental impact, and reaction yields. Regulatory bodies, including those working on environmental health, rely heavily on these details to set safe exposure limits. Taking shortcuts with chemical facts risks people’s health, property, and the planet.
Moving Forward with Reliable Data
There are a few things labs and companies can do to prevent mistakes. Fact-checks and regular training sessions help keep everyone sharp; I’ve been in rooms where knowledge gaps cost time, money, and reputation. Labeling chemicals with both name and formula leaves less room for confusion. Software and digital tools, when integrated with existing procedures, make molecular calculations faster and more foolproof.
Clear, concrete details breed confidence and reduce risk. As chemistry continues to blend into everything from medicine to electronics, a hands-on attitude about formulas and weights saves more than just money—it builds a reputation for responsibility and reliability.
Getting to Know the Chemical
2,5-Dimethylbenzenesulfonic acid pops up mostly in chemical labs and some industrial applications. If you peek at the label, you’ll see a white powder. Smell doesn’t really hit, but that shouldn’t fool anyone—this isn’t something you want drifting around the kitchen or playroom.
Health Risks and Exposure Pathways
Touching, inhaling, or swallowing this compound raises some concerns. Skin or eye contact might cause burning or irritation. If it gets in the eyes, that stinging pain isn’t something anyone forgets. Inhaling any dust can irritate airways.
Research on long-term effects in people hasn’t filled libraries yet, but there are clear red flags when it comes to strong acids and chemical irritants. Reports from lab incidents make clear that accidents with powder forms of chemicals like this can bring on coughing, sore throat, and eye pain. There’s no hard evidence of cancer risk, yet the material data sheet flags this acid for its low-to-moderate acute toxicity.
Environmental Impact Matters
Runoff into water can cause problems for aquatic life. Acidic compounds like this one lower water pH, harming the health of fish and the small organisms they rely on. If factories dump even limited amounts into rivers, the ecosystem takes a hit. Warnings from regulatory agencies place strong emphasis on wastewater controls.
Rural areas near unregulated chemical plants often face strange smells and dying plants. Communities report fish kills downstream from facilities, not always knowing the cause. Water and soil both absorb more than we imagine, which gives the discussion about disposal some teeth.
Worker Safety and Handling
Those who move or use this compound at work stand at the front line of risk. Gloves, goggles, and proper ventilation should show up in any responsible work plan. In my own lab days, carelessness near strong acids led to skin blisters—something that changes your habits fast.
Regulations from OSHA and European bodies set basic expectations. Employers must train workers not just to avoid direct contact, but also to handle spills swiftly. Emergency showers and eyewash stations aren’t optional. Warnings mean something when you hear the stories of chemical exposure firsthand.
Safe Use and Solutions
Minimizing risk starts with proper labeling and training. Too often, stories of chemical accidents start with someone using the wrong container or skipping goggles—something I saw once, with quick regret. Storage in dry, sealed containers and keeping acids away from metals sharply reduces the chance of a mishap.
For waste, neutralization before disposal gives one more layer of defense. Local laws often spell out safe discharge volumes. Community watchdog groups have stepped up—especially in regions where illegal dumping poisoned water. The push to find greener alternatives, even for standard industrial processes, comes from this constant balancing of convenience and harm.
Learning more about household and occupational chemicals—knowing what labels mean, why gloves matter, and how disposal works—keeps people safer in backyards and in factories. Chemicals that seem ordinary on a benchtop shape air, water, and the bodies of those around them. Responsible practices, oversight, and real-world awareness all shape the outcome.
References
Centers for Disease Control and Prevention (CDC). European Chemicals Agency (ECHA). U.S. Occupational Safety & Health Administration (OSHA). Material Safety Data Sheet (MSDS) for 2,5-Dimethylbenzenesulfonic acid.
Understanding the Risks
Working around chemicals isn’t something I take lightly, especially ones like 2,5-dimethylbenzenesulfonic acid. Besides the name being quite a mouthful, this is a sulfonic acid. Many who have done time in a lab or a chemical plant will remember the sting of mishandling such items – from minor skin burns all the way up to more alarming reactions. Safety comes from respect for the risks, and that respect starts with how these acids get stored and how they’re moved around the workplace.
Why Proper Storage Matters
I still remember one old storeroom where acids shared shelves with random solvents and didn’t even have absorbent trays beneath them. That storeroom was begging for trouble. With 2,5-dimethylbenzenesulfonic acid, moisture, heat, and incompatible chemicals set the stage for accidents. Storing it in a cool, dry, and well-ventilated spot reduces the chance of dangerous fumes or the chemical breaking down. I’ve always found that secondary containment – a simple plastic tub, for example – pays off when someone bumps a bottle or a cap isn’t screwed on tight.
Glass or high-grade plastic containers handle sulfonic acids well. Metal cans are a no-go because acids corrode even tough alloys, and no one wants acid dripping through a rusty seam. Clear labeling is another practice I push hard. In busy labs, bottles change places, people change shifts, and guessing games with chemicals aren’t worth the trouble.
Protecting Yourself and Your Team
Handling 2,5-dimethylbenzenesulfonic acid calls for protective gear every single time. I’ve seen colleagues skip gloves thinking they’ll be careful, only to wipe acid off a table with their bare hands moments later. Nitrile or neoprene gloves, splash-resistant goggles, and a long lab coat cover the basics. For larger pours or spills, a face shield and chemical-resistant apron are worth the investment. Eye wash stations and emergency showers must always work and stay easy to reach.
Spill Response and Waste Management
In every place I’ve worked, the best teams drilled for spills. A spill kit, stocked with absorbent material, neutralizer, and cleanup tools, hangs close to the storage space. If something goes wrong with 2,5-dimethylbenzenesulfonic acid, neutralizing agents like sodium bicarbonate reduce the hazard so the mess can get cleaned up safely. Good ventilation clears up any fumes, and it feels reassuring to know a fume hood is ready for transfer jobs or measurements.
Disposal takes discipline, too. Pouring chemical waste down the sink never solves the problem – it just turns it into someone else’s mess downstream. Certified hazardous waste handlers pick up spent acid and used containers. That way, workers stay safe, and local water supplies don’t catch the fallout.
Building a Culture of Safety
Unexpected accidents usually come from shortcuts. Shortcuts come from pressure or overconfidence. Teams I respect build checklists, share information, and never shame someone for asking about safety. Labels, up-to-date safety data sheets, and regular inspections help keep everyone sharp.
The industries that handle 2,5-dimethylbenzenesulfonic acid – whether in research, resin production, or specialty chemistry – do better when people look out for each other. That idea sticks with me more than any regulation: individuals caring enough to do things right, every single time.
A Closer Look at Solubility
Pouring time into chemistry labs and industry jobs, certain patterns with sulfonic acids stand out. Throw a couple of methyl groups on a benzene ring, as in 2,5-dimethylbenzenesulfonic acid, and you start to get a feel for its tricks. The acid’s sulfonic group gives it a hydrophilic punch. Drop some powder into water, you won’t watch it linger for long. It dissolves fast, very well. Expect its solubility to beat out many carboxylic acids and even straight-chained hydrocarbons. Here, that extra sulfonic acid group shoves it into the “easily water-dissolved” category.
Try the same thing in organic solvents like ether or hexane, and the story flips. The strong sulfonic group doesn’t fit in with nonpolar liquids. The result: barely any dissolving. In practice, you’ll see most folks using 2,5-dimethylbenzenesulfonic acid for water-based processes, not oil or solvent-heavy mixes. If you need sulfonation or pH-sensitive reactions in water, this compound steps up reliably.
Physical Properties in the Real World
Pulling experience from bottle to beaker, physical appearance counts for more than just aesthetics. 2,5-dimethylbenzenesulfonic acid comes in a solid, crystalline form, typically white or sometimes with a light tint. The powder packs well, which helps for weighing even in small batches. Handling is straightforward, but this acid, like many sulfonic forms, brings some bite. Contact with skin or eyes feels uncomfortable, so gloves and goggles remain non-negotiable if you want to avoid regrets.
Even in a dry storage room, you’ll notice this acid likes to pull water from the air—hygroscopic is the technical word. Leave a lid off, and the powder clumps. Throw it in a humid warehouse, and you can expect some mess on your hands. Smart storage uses airtight bottles with desiccant packs. Don’t just leave it near open water or steam. From personal lab routine, a careless moment means tossing out a batch because it won’t weigh out correctly anymore.
Why These Traits Hold Weight
Beyond academic charts and tables, these properties lead to direct consequences on the job. For a formulator at a detergent company, 2,5-dimethylbenzenesulfonic acid’s high water solubility means easier mixing without exotic blending tricks. In dye manufacturing, the acid’s brisk reaction in water lets engineers design processes that save energy and cost. Disposal and spill-response come simpler—cleaning up with water neutralization beats battling oily or persistent chemical stains.
That said, safety and environmental impact ride shotgun. In big quantities, sulfonic acids put stress on wastewater treatment, if you’re not careful. Understanding how this acid reacts with metal pipes and fittings keeps you out of corrosion headaches. Some researchers point to advances in resin-based recovery of sulfonates to keep effluents low and recycling high.
Solutions for the Challenges Ahead
Moisture management stands out. Storing 2,5-dimethylbenzenesulfonic acid in climate-controlled rooms with humidity alerts turns clumpy waste into a rare mishap instead of a daily battle. For operations near people, local venting and protective gear go a long way; inhalation or splashes create problems far faster than lab paperwork moves.
On the sustainability front, closed-loop recycling for sulfonic acid streams earns attention both for cost and compliance. Companies pragmatically lean on in-house neutralization tanks, with engineers monitoring pH swings in real time. Investing in R&D for bio-based synthesis can hedge risk as environmental rules tighten. Mixing bench know-how with watchdog science keeps production nimble and safe.