2,4-Difluorobenzylamine: A Deep Dive Into Its Role in Modern Chemistry
Historical Development
Chemists originally sought more reliable aromatic amine building blocks long before 2,4-difluorobenzylamine came along. Interest in organofluorine chemistry surged during the twentieth century, especially as pharmaceuticals incorporating fluorine atoms started showing heightened metabolic stability, improved solubility, and tunable electronic properties. Labs discovered that swapping hydrogen atoms for fluorine atoms on benzene rings brought striking effects on the reactivity and properties of resulting compounds. Once basic amines, like benzylamine, became widely available, researchers experimented by adding fluorines at strategic locations. By the late 1970s, 2,4-difluorobenzylamine emerged as a distinct synthetic intermediate, quickly finding attention among medicinal and crop-protection chemists. The compound grew from a specialty building block into a key raw material for research and industrial pipelines across continents.
Product Overview
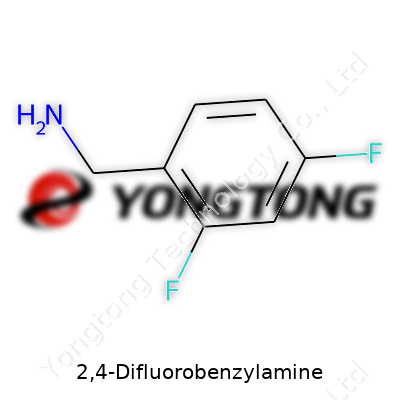
2,4-Difluorobenzylamine stands as an organic compound prized for its ability to introduce the difluorinated benzyl motif. Its core, a benzene ring with two fluorine atoms at the 2 and 4 positions, pairs with a primary amine attached to a methylene linker. Labs and catalog suppliers stock this colorless to pale yellow liquid or solid for projects demanding electron-withdrawing effects. The growing interest in fluorinated pharmacophores owes a lot to 2,4-difluorobenzylamine’s reliable amine group—its versatility and reactivity make it a staple ingredient for drug design, fluorinated agrochemicals, and materials innovation.
Physical & Chemical Properties
2,4-Difluorobenzylamine (C7H7F2N) offers a formula weight around 143.14 g/mol, and in pure form presents as a low-melting solid or lightly oily liquid depending on storage. Its boiling point sits close to 212 °C, and it offers moderate solubility in water, much higher in common polar organic solvents like ethanol, dimethylformamide, and dichloromethane. The molecule carries a faintly amine-like odor, hinting at its basic nature. Inclusion of fluorine atoms imparts high thermal stability, resists oxidative breakdown, and tweaks its interaction with enzymes and metabolic pathways, drawing interest for medicinal chemistry. This compound holds up well under atmospheric exposure during ordinary lab use, but contact with reactive acid chlorides or dehydrating agents demands sensible care.
Technical Specifications & Labeling
Typical product specifications run above 98% purity for research and pharmaceutical applications, with labeling that lists batch number, date of manufacture, and confirmed minimum purity. Reputable suppliers verify the amine content by NMR, GC-MS, or HPLC, ruling out major impurities or isomeric by-products. Labels include standard R and S hazard phrases, alongside relevant pictograms if required by regulatory frameworks like GHS or REACH. Proper labeling provides traceability for any downstream research or regulatory submissions, so labs can track their batches, especially in patent-driven fields.
Preparation Method
Synthesis begins with difluorinated benzyl halides—often 2,4-difluorobenzyl chloride or bromide—reacting with ammonia or an ammonium salt. This nucleophilic substitution converts the benzyl halide to the corresponding amine under mild or moderate heating, and teams often pick solvents like ethanol or acetonitrile to keep the reaction running smoothly. Purification via vacuum distillation, chromatography, or recrystallization delivers the isolated product. Experienced chemists handle the starting halide with proper ventilators and gloves, since its irritant properties exceed those of the finished amine. Industrial producers scale these steps up with continuous flow reactors for reliability at every run, while academic groups adapt the same routes for small-batch or gram-scale needs.
Chemical Reactions & Modifications
The benzylamine functional group performs as an all-purpose nucleophile, eager to engage in acylation, alkylation, sulfonation, and urea formation. Medicinal chemists attach protecting groups, build up amide linkages, and even convert the amine into isocyanates or imines for further ring-building strategies. The difluorinated aromatic ring sets it apart, as the electron-withdrawing fluorines slow electron-rich substitutions but open doors to unique cross-coupling chemistry, such as Suzuki or Buchwald–Hartwig reactions. Variations in the synthetic pathway allow substitutions at the sidechain or the aromatic core, and the compound’s solid amine backbone resists oxidation under most bench conditions. Researchers capitalize on the ring’s stability to access new heterocycles, bridging fragments, or tailor-made ligands for metal-organic frameworks.
Synonyms & Product Names
In catalogs and regulatory libraries, 2,4-difluorobenzylamine also appears as Difluorobenzylamine, 2,4-Difluoro-1-benzylamine, or by its IUPAC identifier: (2,4-difluorophenyl)methanamine. CAS Number 455-68-5 remains the key lookup tag for MSDS sheets, patents, or major chemical distribution platforms. Brands and stockists choose short-hand codes, such as DFBZ-amine or 2,4DFBA, to streamline orders, but the chemical itself invites little ambiguity—its systematic name spells out its unique structure.
Safety & Operational Standards
Working with 2,4-difluorobenzylamine calls for gloves and splash goggles, since contact with skin or eyes can provoke irritation. Vapor exposure at standard lab temperatures remains minimal, but closed storage in cool, ventilated cabinets blocks slow oxidative degradation and keeps the compound from contaminating adjacent materials. Practically all chemical hygiene plans recommend using a fume hood while weighing or transferring samples. Waste solutions containing this amine need segregation from strong acids or bases, and the empties undergo triple rinsing before heading to chemical waste streams. Spills rarely present acute risk, yet even minor exposures should prompt skin and eye washes, as with other small-molecule amines. MSDS documentation, required for compliance, walks users through stepwise response in the event of larger leaks or unplanned exposures, ensuring all staff keep risks under control.
Application Area
2,4-Difluorobenzylamine’s main calling lies in pharmaceutical and agrochemical explorations, where it becomes a scaffold for bioactive molecules—especially those that need metabolic pep from strategically placed fluorines. It lends its amine motif to anti-infective, anticancer, and CNS-active compounds, finding value as a ready-made plug-in for medicinal chemists chasing improved lead compounds. Polymer scientists also nibble at its synthetic edge, working it into monomers for specialty plastics. Some teams tap into its ability to tweak electron density on catalysts and ligands for organic transformations. Its place in the fine chemicals world is secure, offering both a distinctive electronic profile and reliable reactivity with many functional groups found in modern discovery chemistry.
Research & Development
Labs worldwide keep pushing the limits of small-molecule drug discovery, and variations on the 2,4-difluorobenzylamine scaffold stand at the center of structure–activity relationship studies. Explorations often focus on how moving one fluorine or swapping in similar amines affects binding at biological targets. Medicinal chemistry groups screen these analogues in assays for kinase inhibitors, antivirals, and enzyme-blocking drugs, studying absorption, distribution, metabolism and excretion via animal and cell-based models. At research institutions, new coupling methods and greener synthesis protocols explore how to crank out the amine with less waste, higher selectivity, or under milder conditions. The dual fluorine substitution pattern keeps showing up in patent filings, pushing manufacturers and entrepreneurs to devise more efficient and more sustainable industrial routes.
Toxicity Research
Most amines pose moderate risk on ingestion or inhalation, and 2,4-difluorobenzylamine seems no exception so far. Tests in classic mammalian and aquatic toxicity models flag mild to moderate risk, especially for concentrated exposures over hours to days. The difluorinated ring, though stable during regular handling, breaks down in biological systems at rates linked to the overall molecular framework—a fact watched closely by regulatory agencies and industry toxicologists. Eye and skin irritation are the main hazards flagged for most routine lab and process-scale uses. For full-scale pharmaceuticals, extensive ADMET studies dig into every potential pathway and byproduct, checking for mutagenic or carcinogenic potential. To date, no catastrophic incidents or regulatory bans have shadowed the compound, but labs treat every new amine building block with an eye to the long-term, low-dose exposures not always caught in initial screens.
Future Prospects
2,4-Difluorobenzylamine’s value in medicinal and materials chemistry keeps growing as projects push for smaller, more potent, and stable molecular scaffolds. Industry insiders see opportunity in next-generation drugs with improved metabolic control and resistance to breakdown by the body’s enzymes, and difluorinated amines give medicinal chemists one more lever to pull in tuning both pharmacokinetics and pharmacodynamics. Sustainable production methods—like biocatalytic aminations or safer, greener halide substitutions—promise to lower the environmental burden, opening doors to wider applications without ramping up unwanted byproducts. Altered analogues with custom-tailored sidechains sit on the horizon of chemical research, with pharmaceutical and specialty-chemical pipelines testing these structures in animals, plants, and new functional materials. The future for this compound will turn on both its ability to meet tough regulatory and toxicological benchmarks, and its continuing appeal as a unique fragment in the molecular playground of tomorrow’s chemists.
More Than Just a Chemical
Every time scientific names pop up on ingredient lists, most people tune out. Chemical compounds sound far removed from daily routines, but they're working behind the scenes in ways you'd never notice. 2,4-Difluorobenzylamine might fall into that forgettable category, but the story behind this compound offers a glimpse into the careful planning that goes into everything from pharmaceuticals to agricultural solutions.
Building Block for Pharmaceuticals
Pharmaceutical researchers spend years refining drug candidates, and many depend on specialty compounds to get results. This particular chemical stands out as a favorite building block. Synthetic chemists use it for making drugs that treat everything from mental health conditions to infections. The structure of 2,4-Difluorobenzylamine means it can slide right into targeted reactions, creating molecules that fight bacteria or stabilize mood disorders.
My own time in a university research lab taught me how finicky some reactions can get. Certain chemical fragments speed up synthesis, making the whole process more effective. 2,4-Difluorobenzylamine, with its two fluorine atoms, brings a unique twist to its carbon ring structure. That twist comes from fluorine's strength. Drug designers prize it because it helps drugs last longer in the body, leads to better absorption, and can even reduce side effects.
The Crop Protection Side
Not every chemist works in pharmaceuticals. Some turn to agriculture, fine-tuning ways to keep crops safe from pests and disease. 2,4-Difluorobenzylamine plays a role there too. Chemical engineers can use it to develop herbicides and fungicides. These aren’t the kind of broad, old-fashioned chemicals that wipe out everything in sight. Modern pesticides aim to target pests with surgical precision, and these specialty amines help bridge that gap. The goal is to reduce harmful runoff and keep beneficial organisms around, benefitting both farmers and surrounding communities.
Why Safety and Transparency Matter
Trust in science grows when people can see the effort behind these discoveries. The debate over chemical safety keeps making headlines, and I’ve seen the tension up close. In agriculture, anxiety over residues or environmental impact shapes every decision. Drug development faces even tighter scrutiny. Regulators demand tough testing before anything reaches the public, and this extends right down to each building block like 2,4-Difluorobenzylamine. Many compounds, including this one, get evaluated not only for how they behave in final products but also how they’re made and what byproducts result from production.
Staying transparent builds confidence. I appreciate scientists and manufacturers who publish their findings and share data around safety, efficacy, and environmental impact. With open data, health professionals and farmers get the facts, not just marketing claims.
Pushing Toward Greener Chemistry
A smarter future depends on chemists improving their playbook. Too often, industrial processes spill more waste than they should. There's a push for greener chemistry: using renewable raw materials, recycling solvents, and minimizing dangerous byproducts. People working to make compounds like 2,4-Difluorobenzylamine cleaner set a higher standard for the industry. Academic and industry labs need to keep sharing new routes that cut energy use and reduce pollution. I’ve watched a simple change in reaction setup cut our waste to a third of what it used to be, so every step forward adds up.
By understanding 2,4-Difluorobenzylamine’s role, people gain a window into the careful balance between scientific progress, health, and environmental responsibility. Scientists, manufacturers, and consumers all stand to benefit from keeping that conversation alive.
Breaking Down the Structure
Fluorine atoms bring a unique twist in chemistry, especially in organic molecules. With 2,4-difluorobenzylamine, the story starts on a benzene ring. That’s a simple hexagonal ring of six carbon atoms. Place fluorine substituents at spots 2 and 4, and you already set the molecule apart from its monotone cousins. Attach a -CH₂NH₂ group at position one, and you have an amine derivative worth noting.
Chemical Formula: C7H7F2N
Take a closer look: seven carbon atoms, seven hydrogens, two fluorines, and one nitrogen. With this little bit of information, chemists across the globe recognize the basic scaffold they’ll use. Unlike standard benzylamine, these extra fluorines shift the chemical behavior, from electron-withdrawing effects to altered metabolic profiles in pharmaceutical labs.
Why Fluorine Matters
Small tweaks on a molecule can lead to big differences in real-world applications. Adding two fluorine atoms transforms simple benzylamine into something more effective or, sometimes, safer to use. Fluorine’s electronegativity influences bonds nearby, which changes how enzymes interact with the compound or how quickly the body clears it. Drug designers often choose fluorine to slow down metabolic breakdown, letting medicines last longer or activating them at just the right moment.
Touching on Real Uses
Being part of pharmaceutical synthesis, this compound gets used in crafting more advanced molecules—some that go on to treat health issues. Not all chemical modifications work out; some ideas fail in the lab or during animal studies. 2,4-Difluorobenzylamine, though, sits on a short list of popular building blocks.
I’ve watched colleagues in research settings mix 2,4-difluorobenzylamine with other reagents, chasing new patent opportunities. The fluorine atoms can block off parts of the molecule from enzymes that might chew up a medicine too fast, or they can direct a chemical reaction down a different path. These are not just theoretical benefits; companies pore over hundreds of candidates, and those small modifications often mean the difference between a hit compound and failure.
Problems Faced and Ways Forward
No process is perfect. Producing fluorinated aromatics can generate hazardous waste, which rarely gets much attention in company press releases. Chemists navigate tough choices: boosting yields can mean more complex purification, which can lead to higher solvent use and disposal issues. Responsible laboratories have switched to greener solvents and pay extra for proper waste handling, reducing impacts on surrounding communities.
Creative solutions will keep showing up. For example, directed ortho-metalation and modern cross-coupling reactions let chemists add fluorine atoms exactly where they want. Universities and industry researchers design catalysts and methods that lower costs and shrink the environmental footprint. Local regulations often nudge this work along, but it's personal ethics that drive many in the lab to ask tough questions before scaling up.
Importance for Learning and Industry
Students encounter 2,4-difluorobenzylamine in advanced organic chemistry lessons, not just for its textbook formula but as a real-world example of how decisions in the lab ripple outward. Its structure, C7H7F2N, reminds us that each atom counts. Picking up on these small details means safer compounds, better products, and healthier outcomes outside the lab.
Staying Safe with 2,4-Difluorobenzylamine
A bottle of 2,4-Difluorobenzylamine doesn’t look scary, but I’ve seen seasoned chemists pay close attention every time they uncap it. This isn’t just respect for the unknown—years in the lab teach you what happens when someone decides to cut corners. Anyone working with chemicals, especially ones with unknown long-term effects, learns to prioritize personal safety. 2,4-Difluorobenzylamine lands squarely in that category. Skin contact, accidental inhalation, or splashing in the eyes can lead to irritation, and chemical allergies show up when least expected.
I remember a tech who skipped goggles one afternoon—he thought he’d only be pouring diluted solutions. He spent the rest of the day at the campus clinic with red, burning eyes. That incident convinced more than a few coworkers to snap their face shields into place, no excuses. Standard gear—lab coat, gloves, goggles—becomes second nature after experiences like that. Chemically resistant gloves, not those thin ones from the supermarket, give enough protection, and anyone handling the liquid should swap them out if a spill touches skin. Nitrile or neoprene models, for example, limit risk.
Ventilation and Safe Storage
Ventilation matters, too. The fumes from 2,4-Difluorobenzylamine don’t always have a strong smell, so it’s easy to forget about airborne exposure. Fume hoods aren’t just for show; they exist to catch vapors that might build up unseen. It’s easy to overlook, but repeated low-level exposure can add up over time. Take the cap off in a fume hood, make solutions in there, and keep the bottle closed tight unless you’re using it right away.
One frustrating thing about handling chemicals like this is how quickly a careless storage habit can cause trouble. I’ve opened fridges to find vials jammed next to food, or left open overnight on a bench. Every container ought to have a clear label, including hazard information. Good labels prevent a mix-up when people rotate through a lab. Storing 2,4-Difluorobenzylamine in a cool, dry place, away from acids and oxidizers, keeps it from decomposing or reacting with neighboring chemicals. Regular checks of storage areas cut down on surprise leaks or unexpected damage to containers.
Dealing with Spills and Accidents
Spills demand immediate action. I’ve seen panic in the eyes of new lab members when a bottle tips. A spill kit—absorbent materials, neutralizing agents, disposable scrapers—proves its worth fast. Scrub down any area where the chemical touched, toss cleaning materials into dedicated waste bins, and wash hands before touching anything else. Doing dry runs with spill kits when no one is stressed builds muscle memory.
Most importantly, nobody should work alone with chemicals like 2,4-Difluorobenzylamine. I’ve relied on colleagues to help with eyewash stations and emergency showers. Someone else watching your back often makes the difference if something goes sideways.
Training and Good Habits
Training goes beyond reading a single safety sheet. Labs that run regular refresher sessions on chemical hygiene keep problems down. I’ve noticed new trainees who take safety drills seriously tend to avoid unnecessary risks—confidence grows with practice. Listening to stories from older coworkers about “the day someone forgot their gloves” brings the lessons home in a way that books and online modules never quite capture.
The main thing with 2,4-Difluorobenzylamine: treat it with respect, know its health risks, and build safe habits into everyday routines. Respecting chemical safety doesn’t slow down productivity—it keeps the whole team standing, eyesight intact, and hands free from burns or rashes. That’s worth more than cutting a single corner for speed.
Understanding the Substance
2,4-Difluorobenzylamine shows up in many research labs as both a building block in organic chemistry and in pharmaceutical development. Handling chemicals isn’t just about ticking boxes. I’ve spent enough time at lab benches to know how easy it is to cut corners—until something goes wrong. This compound brings with it a set of risks that demand some respect and a strong storage protocol.
Why Proper Storage Makes a Difference
Chemicals like 2,4-Difluorobenzylamine don’t announce their presence in the air or offer clear signals of danger. Just because a bottle looks clean and sealed doesn’t mean the contents aren’t volatile, reactive, or harmful. Accidents in storage areas almost always come down to two things: improper container sealing or mixing incompatible substances. Both have the potential to ruin a work day—or worse, impact health and safety.
Key Considerations for Safe Storage
Flammable liquids make up a big category of lab accidents. 2,4-Difluorobenzylamine won’t ignite spontaneously, but store it near a heat source or in sunlight, and you’re asking for trouble. Store it in a cool, dry space, away from direct sunlight, heaters, radiators, or any unpredictable temperature fluctuations. Heat can increase the vapor pressure in bottles, leading to leaks or, even worse, a burst container.
Avoid storing it with oxidizers, acids, or bases. I’ve seen the result of mixing incompatible materials: chemical burns, toxic vapors, ruined experiments. Even trace contamination can trigger reactions that compromise more than just a batch of product—they threaten everyone sharing the lab.
Don’t Skimp on Containers
2,4-Difluorobenzylamine tends to eat away at plastics. Glass containers with tight, chemical-resistant caps work best. If the supplier ships it in a specific bottle, that’s for a good reason. Pouring chemicals into a new container just for convenience can open the door to leaks or spills.
Labels seem like an afterthought to busy researchers. Skipping them leads to confusion or, worse, misidentification. I keep a permanent marker next to my storage area for exactly this reason. The chemical name, date received, and hazard symbols should go front and center. If there’s ever an emergency, responders lose precious seconds if information’s missing.
Protecting Ourselves and the Environment
Every bottle needs a secondary containment tray or bin—especially those holding organic amines like this one. If a leak develops, the tray contains the mess. No one wants to deal with hazardous spills on shelves or floors, especially in tight lab spaces, and cleanup gets much simpler. Absorbent pads, chemical spill kits, and personal protective equipment like gloves and goggles are my regular companions for a reason: unexpected splashes or fumes have lifelong consequences.
Training and Checklists
I’ve seen new lab members forget to check expiration dates, skip inspecting seals, or overlook changes in color or odor. Regular reviews of safety data sheets (SDS) and refreshers on best storage practices stop complacency from setting in. A simple checklist on the door or wall, updated every semester, can catch these easy-to-miss errors before they snowball into trouble.
Final Thoughts on Responsibility
Safe chemical storage comes down to personal responsibility and good habits, not just regulations. Neglect invites risk, and the costs hit more than your own work. A safer storage plan for 2,4-Difluorobenzylamine means fewer emergencies and a sturdier foundation for research that pushes science forward.
Understanding Purity in the Chemical World
Anyone in research, manufacturing, or pharmacy appreciates the importance of knowing exactly what’s inside their bottles. Purity isn’t just a lab metric; it shapes experiments, outcomes, and sometimes safety. 2,4-Difluorobenzylamine, like most specialty chemicals, gets sold at a range of purity levels. These numbers matter especially for chemists synthesizing pharmaceuticals or developing analytical methods, where even a small impurity might ruin data or add unexpected risks.
Industry Standards and What’s Out There
Most suppliers list this material at 97% or 98% purity, with the highest grades reaching 99%. On paper, the extra percentage point might seem minor, but in practice it can mean the difference between a reaction that works cleanly and one that throws off a batch. Looking at Sigma-Aldrich and Tokyo Chemical Industry – two giants in laboratory chemicals – their catalogs typically offer 2,4-Difluorobenzylamine in ranges from “analytical grade” (97%) to “high-purity” (99%). These labels reflect extensive analytical screening, including NMR, GC-MS, and HPLC, rather than just taking the manufacturer’s word.
Not every user needs 99% though. My years working in academic labs taught me that basic synthesis projects get by just fine with 97–98%. We reserved ultra-high-purity only for the toughest jobs, like making reference standards or formulating something for human trials. On the other hand, process engineers in pharma will pay for 99%+ every time—no shortcuts tolerated. Accuracy trumps cost once the stakes get high enough.
Why Purity Isn’t Just a Number on a Label
Purity shapes risk, trust, and both cost and performance. Low-level contaminants may go unnoticed if your analysis tools aren’t sensitive, but their effects can pop up later. I once ran a pilot project using a 98% batch, only to have our analytical chemist spot a chlorinated by-product at 0.2%. Theoretical yields looked fine, but downstream tests flagged it as a potential genotoxic impurity. The lesson stuck: even “small” impurities can have a big impact.
For someone buying this chemical, the conversation with the supplier often digs deeper. Reputable companies offer certificates of analysis (COA) listing not only the headline number but also individual impurities and water content. If the seller refuses or the documentation looks thin, that’s a huge warning sign. For labs operating under GMP or GLP, audit trails and validated analytics become the baseline.
Navigating Purity and Procurement
Anyone purchasing 2,4-Difluorobenzylamine should ask for batch-specific COAs, not just generic spec sheets. Third-party analytical data, where available, adds a layer of security. Some research groups set up in-house confirmation, especially if the product is a key part of a multi-step synthesis or destined for preclinical studies. Proper handling and storage matter too; even pure chemicals degrade when left near sources of heat or moisture.
Cost can push buyers toward the lowest-acceptable purity. That makes sense for basic tests or teaching labs, but the price difference between 97% and 99% often shrinks against the cost of failed experiments or regulatory headaches. I always recommend budgeting for the highest purity directly relevant to your project’s risk profile. Cutting corners here can trip you up further down the line.
Final Thoughts on Getting the Most from Your Chemical Investment
Modern supply chains mean options exist for every budget. Still, it pays to verify claims, insist on thorough documentation, and match the purity to the project. The upfront effort in selecting and confirming purity levels for 2,4-Difluorobenzylamine repays itself in reliable outcomes and fewer unwelcome surprises.