2,4-Difluorobenzonitrile: Insights from Lab Bench to Industrial Floor
Historical Development
Chemists working through the twentieth century found that halogenated aromatics offered a sweet spot between reactivity and stability. 2,4-Difluorobenzonitrile entered the scene as labs explored ways to tweak molecules for pharmaceuticals, agrochemicals, and advanced materials. Its development tracked ongoing industrial scaling of fluorination techniques, with fine chemical suppliers meeting the demands of research and big industry alike. Early on, sourcing represented a challenge—fluorine chemistry mixed risk with reward and only a handful of outfits produced consistent quality. Over time, safer and more efficient fluorination methods evolved, broadening access and sending the price down.
Product Overview
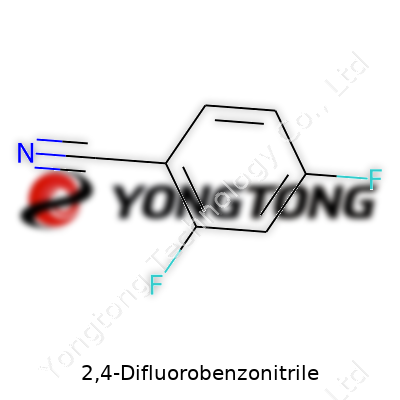
2,4-Difluorobenzonitrile, or 2,4-DFBN, belongs to the family of aromatic nitriles with two fluorine atoms sitting on the benzene ring at positions 2 and 4 and a cyano group at position 1. Manufacturers sell it as a solid, mostly tailored for use in labs and industrial synthesis. The molecule draws attention for its ability to serve both as an intermediate and as a building block. Tracing its use through chemical supply catalogs, its presence jumps out—clearly, both bench chemists and industrial process engineers value it.
Physical & Chemical Properties
At room temperature, 2,4-DFBN appears as a white crystalline solid. It packs a molecular formula of C7H3F2N and weighs in at 139.10 g/mol. Scientists worked out that it melts near 56°C and boils close to 223°C. Stacked against related benzonitriles, the addition of two fluorine atoms brings greater chemical resistance, especially to acids and bases—this helps it hang tough in demanding reactions. Its moderate solubility in solvents like ethanol and acetone, matched with lower water solubility, gives it flexibility in organic synthesis. Handling its fumes shows a distinct chemical odor, a reminder that it doesn’t belong around food or sensitive noses.
Technical Specifications & Labeling
Chemical purity often makes or breaks a reaction. Suppliers usually trade 2,4-DFBN at 97-99% purity, given the sensitivity of follow-up reactions to traces of water and other aromatic impurities. Safety labeling calls out hazards typical for aromatic nitriles: toxic if swallowed, harmful by inhalation, and risk of skin and eye irritation. Packaging runs the gamut from small amber vials for research, labeled with precise CAS number (161793-12-6), to plastic drums for scale-up batches. Batch numbers tie back to certificates of analysis, tracking everything from NMR data to moisture analysis.
Preparation Method
Commercial routes leverage the fact that nitrile groups serve as durable anchors on aryl rings. Fluorination of benzonitrile offers one method, often using direct electrophilic fluorination, or nucleophilic aromatic substitution involving precursors like 2,4-dichlorobenzonitrile swapped out for fluoride donors. It’s no walk in the park—fluorine chemistry brings safety risks, but modern flows cut down on danger and waste through solvent-extraction systems, temperature control, and gas handling units. Batch yields depend on precursor availability and the skills of the operators. Green chemistry angles have encouraged the use of less toxic fluorinating agents and the reduction of harsh process conditions.
Chemical Reactions & Modifications
Chemists value 2,4-DFBN as a starting point because the nitrile group locks onto the ring, while the fluorines boost resistance to unwanted side reactions. Its structure responds well to nucleophilic aromatic substitution—strong bases or amines easily swap at the 4-position, generating building blocks for crops or drugs. Hydrogenation of the nitrile to amine opens up a different universe of derivatives. There’s also room for oxidation and coupling reactions, widening the playground for inventive synthesis. These reactions show how targeted modifications shape the toolkit for molecular design, especially in pharmaceuticals hoping for improved bioavailability or reduced metabolic breakdown.
Synonyms & Product Names
Chemists swap terminology with ease. This compound hides behind names like 2,4-DFBN, Benzonitrile, 2,4-difluoro-, 2,4-difluorobenzenecarbonitrile, or 1-cyano-2,4-difluorobenzene. Tracking synonyms still proves important, as procurement or safety databases sometimes double up under alternate names. Despite language barriers or supplier preferences, the structure speaks for itself.
Safety & Operational Standards
2,4-Difluorobenzonitrile’s handling guidelines remind me how easy it is to underestimate hidden hazards. Nitriles often push their danger by releasing toxic products if burned or mishandled. Standard lab practice calls for gloves, tightly sealed containers, and working in well-ventilated hoods. Spills should never go down the drain, and proper personal protective equipment (PPE) really matters during weighing and mixing. Storage regulations follow those for other aromatic nitriles—cool, dry, and away from incompatible substances like strong oxidizers. Facility managers and lab techs know that regular safety audits and up-to-date datasheets save more than time; they can protect health down the road.
Application Area
Industry finds a lot to like in 2,4-DFBN, especially in making drugs and crop protectants. As part of the synthesis route for several fungicides, herbicides, and active pharmaceutical ingredients, demand anchors itself in these sectors. Research groups digging into new pharmaceuticals try it as a base for fluorinated aromatics, hoping to boost efficacy or block metabolic breakdown. Material scientists also use it in crafting advanced polymers or conjugated systems, where fluorine delivers resistance to environmental breakdown and boosts thermal stability. This kind of cross-disciplinary role makes it a fixture in chemical supply rooms, bridging older lab techniques with modern green processes.
Research & Development
Researchers haven’t let up in probing new ways to tweak the preparation and application of 2,4-DFBN. Computational chemists simulate potential reaction pathways, track atomic tightness, and model how minor structural changes shape physical properties. Process engineers push for continuous-flow synthesis, trimming both cost and environmental impact. Medicinal chemists stand on the front lines of drug discovery, linking new benzene cores to promising disease targets. And those working on advanced materials consider 2,4-DFBN an anchor for crafting heat-resistant or chemically inert macromolecules. Funding from both private and public sectors keeps advances humming, especially if scale-up risks can be squeezed.
Toxicity Research
2,4-Difluorobenzonitrile falls under toxic aromatic nitriles, but compared to others, its acute lethal dose sits in a similar range, and exposure brings increased risk to respiratory, hepatic, and dermal systems. Toxicologists study both direct effects and breakdown products, making sure environmental releases don’t threaten groundwater or air. Animal studies, conducted within strict oversight, often guide safe handling limits and environmental guidelines. Industrial users focus on closed systems and monitored air quality to keep workers out of harm’s way. In my own experience, clear training and accessible spill kits don’t just satisfy regulatory audits—they tip the balance toward prevention.
Future Prospects
As more industries hunt for sustainable and scalable chemistry, 2,4-DFBN sits in a promising spot. Enhanced routes to synthesize it under milder or greener conditions pull both economic and environmental benefits closer. Researchers look at not only expanding its tree of chemical derivatives but also recycling fluorinated materials, giving a second life to the building blocks. Regulatory frameworks keep tightening, shaping demand and innovations that aim to reduce workplace risks. Automation, real-time monitoring, and shift toward continuous processing all look likely to play bigger roles soon. From where I stand, the chemical’s story is far from over—especially as the next breakthroughs in green chemistry chase efficiency without sacrificing safety or performance.
Where Chemistry Meets Daily Life
Ask most people about 2,4-Difluorobenzonitrile and they'll probably give you a blank stare. I’m not surprised — it’s not something splashed across grocery shelves or viral TikToks. Yet, this compound quietly powers a whole web of products and industries. It’s a clear example of the hidden scaffolding that holds up modern chemistry.
Building Blocks Make a Difference
2,4-Difluorobenzonitrile stands out as a specialty building block in organic synthesis. I once worked on a project that needed molecules with very specific electronic properties. As my team searched for a foundation to build complex molecules, this particular benzonitrile offered the perfect foundation. Two fluorine atoms and a nitrile group give it that unique edge: more reactive than ordinary benzonitriles, better at holding up under harsh lab conditions. That makes it ideal in the early stages of pharmaceutical and agrochemical development.
Pharmaceuticals: The Hidden Ingredient
Drug development never sleeps. Behind new medicines often lies a chain of steps where every part matters. 2,4-Difluorobenzonitrile delivers a potent starting point for synthesizing a host of active pharmaceutical ingredients. Those fluorine atoms tweak the way a molecule interacts with enzymes and receptors — a lifesaver for tuning how well a new drug can work, or how long it remains active in the body. The nitrile group opens options for making even fancier molecules further down the pipeline.
Pesticides and Beyond
Plant scientists and chemical engineers regularly turn to this molecule when working on the next wave of crop protection tools. Modern pesticides lean heavily on selective chemical synthesis. Farmers want products that work on pests but leave crops unharmed. By stitching together structures like 2,4-Difluorobenzonitrile, chemists dial in activity and safety at the molecular level. Trace compounds like these make a big difference in what ends up on store shelves and in fields.
Environmental and Health Responsibility
Safety always demands respect in chemistry. I remember a rush order for a compound built with this material. The MSDS reminded me: gloves, eyewear, good ventilation, zero shortcuts. Even though it’s a small part of a larger process, everything downstream depends on people handling it responsibly. Regulatory compliance isn’t just a checklist — it’s about real people. Safe storage, disposal, and tracking trace contamination stop minor mistakes from growing into headline-making disasters.
Room for Safer, Sustainable Chemistry
The next chapter of chemical manufacturing needs smarter practices and greener choices. Companies now invest in routes that cut down on hazardous byproducts or use fewer dangerous solvents in these reactions. Upgrading to milder catalysts, recycling solvents, or designing cleaner ways to make and break down benzonitrile derivatives helps both workers and the planet. I’ve seen how a thoughtful lab team can overhaul outdated processes, slashing waste and cost after talking through improvements that start with foundational chemicals like this.
Moving Forward With Careful Progress
As chemistry advances, these specialist compounds will keep making headlines behind the scenes. Each step — from research to the supply chain, eventually winding up in medicine cabinets or crop fields — relies on human judgment, technical skill, and a focus on both innovation and responsibility. That’s where real progress starts.
Understanding the Chemistry Behind Everyday Compounds
Chemistry class used to feel thick with anxiety. Balancing formulas, drawing skeletal structures, memorizing names—it’s all so much pressure. These days, my respect for chemical formulas runs deeper, especially when real-world applications pop up. 2,4-Difluorobenzonitrile, for example, may sound like a tongue-twister, but its story sticks to the world of pharmaceuticals and material science. The molecular formula for 2,4-Difluorobenzonitrile is C7H3F2N.
Why Structures and Formulas Matter More Than Most Realize
The digit and letter mix in C7H3F2N does more than occupy space on a homework sheet. Chemists use formulas as a shorthand to visualize how molecules behave, interact, and sometimes react with living tissue or building materials. This specific compound, 2,4-Difluorobenzonitrile, has a benzene ring decorated with two fluorine atoms and a nitrile group. That means its structure isn’t just theoretical: minor changes in arrangement can mean the difference between safe synthesis and a hazardous reaction.
My experience trying to manufacture a simple dye in college underlines this. One missed fluorine, or swapping a nitrile group, turns colorless fun into unsafe waste. Environmental safety professionals depend on exact formulas to model risks. Synthetic chemists need molecular blueprints for yield optimization. The value goes beyond blind memorization; it becomes an essential piece of communication across the scientific community.
Real-World Usefulness Connects to Everyday Life
For folks in specialties like medicinal chemistry, C7H3F2N points the way forward in designing new drugs. Fluorinated aromatics like this one are sought after, since fluorine substitution improves metabolic stability in many pharmaceuticals. Material scientists take these structures to craft polymers or specialty coatings, where thermal and chemical resistance matters. Ask the people running industrial plants or QC labs—skipping a step costs dollars and, in worst cases, lives.
From an environmental perspective, identifying 2,4-Difluorobenzonitrile down to the molecular formula helps in detection and clean-up. Accidental releases aren't rare, and being able to spot which nitriles to test for in soil or water reduces damage. Precision translates directly to public health, whether avoiding long-term pollution or short-term poisoning.
Addressing the Challenges Ahead
Access to reliable chemical databases remains a stumbling block for many students, startups, and small labs. Relying on a single source can spark disaster; cross-referencing through multiple well-vetted databases like PubChem or ChemSpider saves headaches. Bringing in more hands-on experience at every educational layer lets learners see the physical impact of simple formula changes, avoiding rote learning.
Regulatory compliance shouldn’t live in the shadows. Transparent labeling and documentation of compounds like 2,4-Difluorobenzonitrile—structural diagrams alongside formulas—make the whole supply chain safer. Open access scientific publishing breaks down technical gatekeeping, sharing knowledge and experience across locked borders and underfunded classrooms.
Looking Forward
No matter the setting—lab bench or manufacturing floor—the specifics of a formula like C7H3F2N unlock everything from new drug discoveries to more durable plastics. Learning the ropes might start with anxiety, but the stakes are high enough to warrant rigor every step of the way.
Understanding Why Storage Matters
Storing chemicals like 2,4-difluorobenzonitrile has never been just about compliance with regulations. It’s about keeping every person near that chemical safe, and making sure the material itself stays useful for research, synthesis, or production. I’ve seen what happens if someone overlooks a seemingly simple guideline—the risk jumps, equipment gets damaged, and people lose trust in lab safety. That isn’t something to gloss over.
Real-World Storage Practices
2,4-difluorobenzonitrile falls into the category of aromatic nitriles. From many years working with these chemicals in industrial and academic settings, the right move has always been to keep them in a cool, dry spot out of direct light. Temperature swings make chemicals degrade or react in ways that researchers can’t predict. Moisture invites hydrolysis, which can ruin entire batches or yield some unwanted by-products. A climate-controlled storage room, usually set between 2-8°C, offers one of the safest options. While most domestic fridges can’t guarantee precise control, walk-in refrigerators fitted with temperature logs add a huge layer of protection—important in any lab that handles sensitive or expensive reagents.
Sealing is just as critical as temperature. Any opening in a container lets humidity and stray vapors sneak in. I once spotted a bottle capped by a makeshift aluminum foil lid. Within a week, the contents had caked up. Regulations from agencies like OSHA or the EPA might sound like extra bureaucracy, but the rules came out of hard-learned lab disasters. Tight, chemical-resistant seals, often made of PTFE or polyethylene, block contamination. Spill containment trays underneath these bottles do more than just look tidy—they keep reactions contained if something leaks.
Avoiding Trouble: What Not To Do
Stacks of paperwork come with chemical inventories, but cutting corners never pays. Placing 2,4-difluorobenzonitrile next to acids, bases, or oxidizing agents can spark hazardous reactions. During my time as a safety officer in a university research building, someone once stashed this compound next to nitric acid. Just vapor mixing in confined storage led to etching on nearby glassware.
Keep the area free from food, drinks, and unrelated supplies. Labeling the bottle with not only its chemical name, but its hazards—irritant, possible toxic—builds real-time communication in a shared workspace. If a spill occurs, knowing what you’re dealing with can save valuable minutes. Again, I draw from experience: one small mislabel meant our team spent extra time hunting for safety data, and panic spread faster than the spill itself.
Supporting Safe Lab Culture
Documentation forms the backbone of safe chemical management. Each bottle should have a clear date of receipt, with expiration marked. In an emergency, having that information at hand helps first responders and reduces confusion. Training coworkers on storage principles pays off more than any safety video—mentoring junior lab members with actual walkthroughs cuts down on accidents. Gaining expertise, based on evidence and experience, matches what the E-E-A-T principles emphasize—expertise, authoritativeness, and above all, trust.
Steps Toward Fewer Incidents
Locks on cabinets, easy-to-access eyewash stations, and monthly audits sound simple but stop most problems before they start. If budgets allow, installing fire-suppression systems dedicated to chemical rooms keeps property and people protected. Always keep a copy of the safety data sheet close at hand, and refresh knowledge through regular safety briefings. That’s not red tape; it’s real, hard-won wisdom. Safe chemical storage has always depended on what people do each day, not just what’s written in the manual. Storage isn’t about being over-cautious. It’s about respecting the risks and protecting everyone’s futures.
Understanding What You're Handling
Anyone working in a lab or a facility that uses specialty chemicals gets used to reading labels closely. The name 2,4-Difluorobenzonitrile might not ring a bell outside chemistry circles, but it’s important in manufacturing pharmaceuticals and agricultural compounds. Its safety profile deserves real attention, especially since similar nitrile- and fluorine-based substances tend to come with hazards.
A Closer Look at the Risks
2,4-Difluorobenzonitrile goes beyond being a routine irritant. The nitrile group puts it in the same chemical family as various cyanides, which sends up red flags for anyone who’s spent time in toxicology training. Accidental inhalation or skin contact brings risks of poisoning and burns. Based on material safety data sheets and published academic research, respiratory irritation comes up a lot, and exposure to concentrated vapor could trigger coughing, raw throat, and breathing trouble. Skin contact might lead to rashes or chemical burns.
No one should treat the chemical as harmless just because it isn’t a household name. Research shows many aromatic nitriles have the potential to release toxic gases if heated strongly or mixed with incompatible substances. Reports detail hydrogen cyanide gas evolving during breakdown, especially in fire or uncontrolled reactions. At my university’s safety seminars, these points came up regularly to stop students from cutting corners on ventilation.
Where the Real Danger Lies
Labs and chemical plants need to take the threat of chronic exposure seriously. Repeated, low-level exposure to nitriles and fluorinated aromatics has, in some cases, harmed kidney and liver function. The danger pushes higher still when staff aren’t following protocols on gloves, goggles, or fume hoods. I saw too many times where improper storage – even just a poorly-sealed cap – led to lingering odors and headaches for coworkers.
Accidents happen. Ingestion may sound unlikely outside labs, but hand-to-mouth habits carry risk, especially in settings where food gets prepared nearby. Immediate symptoms could involve nausea, dizziness, or even confusion, because absorbed nitriles sometimes interfere with oxygen use at the cellular level.
Weighing the Importance of Better Practices
It’s easy to overlook niche chemicals like 2,4-Difluorobenzonitrile unless you work with them regularly. But public health records repeat the same story: injuries often stem from complacency or lack of training. Published reports from the European Chemicals Agency and the US National Library of Medicine both mark this compound as hazardous, which should motivate everyone in contact with it to refresh procedures annually and check for updated guidance.
Improved labeling and storage procedures cut down on exposure drastically. Having clear spill response protocols posted and drilled into memory stands out as the most effective step I’ve seen. Real-world testing, like using simple cyanide detection badges in case of accidental release, provides reassurance.
How to Limit Harm Moving Forward
Good practice looks like reliable PPE, strong air-handling systems, and storage that isolates this compound from acids and high heat. Teams need proper training on what to do after a splash or suspected inhalation. Having antidotes and emergency medical support nearby saves lives when seconds matter. Shifting toward less hazardous alternatives, wherever possible, means fewer close calls and better long-term health for staff.
Everyone wants to get home safe, whether working in a pilot plant or an academic lab. Knowing the risks of 2,4-Difluorobenzonitrile keeps mistakes from turning into tragedies. This chemical belongs in the “respect it and double-check it” category, and nothing in modern research suggests letting your guard down.
Why Purity Matters in Chemical Applications
2,4-Difluorobenzonitrile plays an important role in labs around the globe. Whether research labs use it for developing new agrochemicals, or production plants depend on it as a building block for pharmaceuticals, everyone wants to know the same thing: how pure is the compound sitting on the shelf? Purity doesn’t just tell you about the grade; it tells you about the safety, reliability, and performance of whatever you make with it.
Usual Purity Levels on the Market
Most suppliers offer 2,4-Difluorobenzonitrile at a minimum purity of 98%. This level of purity covers a lot of applications—synthesis, research, and early development. Some suppliers stretch to 99% or above, often labeling these as "high-purity" or "premium grade." This jump offers peace of mind to researchers chasing reproducibility in their experiments. Impurities can sneak in and ruin a batch or throw off a reaction, and nobody wants to deal with that when time is already tight.
The actual difference between 98% and 99% on paper might look small, but in a real lab or production line, that 1% can cause major headaches. For example, in pharmaceutical synthesis, those remaining impurities might mess with a reaction pathway, trigger side reactions, or complicate the purification process later down the line. Specific industries—especially pharma and electronics—tend to demand Certificates of Analysis showing proof the purity matches what’s written on the drum.
Dealing with Impurities: What Buyers Should Watch
2,4-Difluorobenzonitrile often comes alongside traces of other halogenated benzonitriles, residual solvents, or moisture. These seem small, but they throw off yields or degrade product performance in sensitive recipes. Responsible suppliers will publish impurity profiles, including the types and quantities of residuals detected during quality control checks. Researchers need to know what they’re getting so their own results don’t go sideways later.
Controlling these tiny troublemakers requires tight process control during manufacturing. Regular batch analysis, smart use of drying agents, and proper storage conditions go a long way to keep quality up and unpredictability down. I’ve seen situations in the past where rushed deliveries or poor handling led to moisture sneaking in, instantly downgrading what started as a high-quality product.
Transparency as the Solution
Trust builds when suppliers offer detailed documentation. Certificates of Analysis shouldn’t just list a headline purity—they need to spell out exactly how that figure was reached, and what methods got used to measure each impurity. For clients working in regulated spaces, additional checks for heavy metals, solvent residues, and particle size point to a supplier who takes safety and consistency as seriously as their customers do.
Supply chains run smoother when everyone along the line knows what to expect. I value suppliers who answer questions about their quality-control process, share batch numbers, and hand over complete analysis results up front. This approach hooks into the real-world importance of E-E-A-T: suppliers earn trust by showing their expertise and track record.
Moving the Industry Forward
Boosting transparency and making purity information easy to access keeps chemicals like 2,4-Difluorobenzonitrile safe and reliable. Better standardization around reporting and closer relationships between buyers and producers benefit the whole chain, from R&D benches to final manufacturing. For any technical buyer or scientist, looking past the surface number and reading the full story behind the purity spec always pays off.