2,4-Difluoro-dl-phenylglycine: More Than a Chemical Structure
Historical Development
Chemists have always searched for building blocks that unlock potential in fields from drug design to new materials. 2,4-Difluoro-dl-phenylglycine grew out of that quest. Early innovation in organofluorine chemistry opened a door, so researchers could introduce fluorine atoms onto aromatic amino acids. By the late 20th century, as industries demanded precision and selective function in their molecular tinkering, this compound joined the growing class of custom-synthesized amino acids. Scientific journals in the late 1990s and early 2000s trace its debut, usually alongside discussions on peptide modification and novel pharmaceutical scaffolds. Now, it represents both a milestone in selective halogenation and an enduring tool for those in need of tailored amino acids.
Product Overview
2,4-Difluoro-dl-phenylglycine doesn’t usually show up in headlines—and it sure isn’t in most undergrad textbooks—but it attracts the hands of people tweaking molecular scaffolds for something new. This synthetic amino acid belongs to the family of phenylglycines, featuring two fluorine atoms at the 2 and 4 spots on its aromatic ring. These modifications can give molecules surprising resilience to enzymatic breakdown, change their physical interactions, and steer biological activity in unpredictable directions. Even a minor change, like swapping out hydrogens for fluorines, keeps drug designers busy because of unexpected shifts in receptor binding and metabolism.
Physical & Chemical Properties
2,4-Difluoro-dl-phenylglycine typically forms as a white to off-white crystalline powder, often described as faintly aromatic, but the real story unfolds in the specs: molecular formula C8H7F2NO2, molecular weight about 187.15 g/mol, melting point often ranges near 180°C. Its solubility leans towards the polar, with water giving middling results and common organic solvents—methanol, DMSO—often preferred for reaction workups or biological screening. Fluorine atoms tweak not just reactivity, but also lipophilicity and hydrogen bonding profiles, which shift analytical retention times and stretch applications a bit further than the unmodified cousin.
Technical Specifications & Labeling
Suppliers list this compound with purity exceeding 98%, since applications demand accuracy. IR and NMR spectra reveal characteristic stretches and chemical shifts, notably sharp doublets from aromatic protons near the substitution points, and upfield shifts for the fluorinated carbons in 13C NMR. Commercial labels typically clarify racemic composition (both D and L forms), batch number, storage conditions, and regulatory status, all to keep chemists and regulators on the same page. Exact quantities—1g, 5g vials—match research needs, while strict labeling keeps hazardous materials info and handling instructions clear on the bench.
Preparation Method
Industrial and academic methods overlap here. A classic route starts with fluorinated benzaldehyde, using Strecker or homologation reactions to add in the aminoacetic acid side chain. Reductive amination also comes into play, tweaking conditions to coax the difluoroaromatic through multi-step synthesis. Each step demands careful control: temperatures up or down, reagents dried, solvents degassed. Chromatography, usually flash silica, sorts the product from by-products. The more ambitious labs throw in chiral catalysts, targeting enantioenriched forms. Each tweak produces subtle changes, but most researchers settle for the achiral blend unless specificity matters downstream.
Chemical Reactions & Modifications
This molecule sees most use in peptide coupling, where its amine and carboxylic acid groups fit neatly into amide bonds. The difluorinated aromatic ring resists oxidation and some metabolic enzymes, so pharmacologists appreciate its ability to hold its shape. Common reactions target the creation of esters, conjugates, or protected derivatives, for easier handling in multi-step syntheses. Fluorine’s strong electron-withdrawing character pulls electron density away from the ring, so electrophilic attack slows, while nucleophilic aromatic substitution becomes more selective. This balance keeps synthetic chemists experimenting with ways to extend, derivatize, or selectively activate the material.
Synonyms & Product Names
Lab vernacular can muddy the waters, as suppliers and journals refer to this compound in different shorthands. Some catalogs list it as 2,4-difluorophenylglycine, others drop the dl prefix or use "αamino-2,4-difluorophenylacetic acid" to hint at its structure. Nobody can standardize lab slang, but the CAS number (386858-51-5) offers a common denominator for unambiguous ordering or regulatory checks.
Safety & Operational Standards
Every gram matters when handling experimental fluorinated compounds. This compound demands standard lab safety: gloves, goggles, and ventilation, since small molecules sometimes pack surprising toxicity. Dust inhalation or skin exposure rank as the main hazards, especially given possible systemic toxicity. Regulatory labeling tracks hazardous potential, with storage in cool, dry conditions away from oxidizers or strong acids as a baseline. Waste and wash solvents require disposal in accordance with local chemical regulations, especially since fluorinated residues persist in the environment longer than many realize. Training and attentiveness form the real shield against workplace mistakes.
Application Area
It takes a crafty chemist to see where difluorinated amino acids shine. Mostly, project teams draw on 2,4-difluoro-dl-phenylglycine for its structural effects in drug discovery projects, using it to toughen peptide drugs against breakdown, alter transport through cell membranes, and tune binding interactions in enzyme assays. Peptidomimetics research gets a lift from hard-to-metabolize analogs, opening possibilities in antiviral or anti-inflammatory therapeutics. Beyond the pharma world, the material occasionally pops up in agrochemical studies or advanced polymer projects, where controlling shape and interaction often brings commercial advantage.
Research & Development
Academic and commercial labs alike crank out papers on the role of difluorophenylglycine in expanding the chemical biology toolbox. Teams explore its effect on metabolic stability, receptor affinity, or protein-protein interactions. Some recent work investigates targeting difficult protein pockets by prepping peptides that resist enzymatic digestion, hoping for longer half-lives and improved specificity. Fluorine’s impact on bioactivity continues to surprise, prompting new rounds of study in computational chemistry and molecular modeling.
Toxicity Research
It’s one thing to make new molecules—it’s another to make them safe. Ongoing toxicity work tracks how difluorinated aromatic amino acids interact with biological systems. Animal testing and in vitro models examine acute toxicity, chronic exposure, and metabolic byproducts. Regulatory bodies want firm answers about bioaccumulation, mutagenicity, and off-target organ effects. Preliminary results show that while low doses present manageable risk in controlled settings, large-scale use calls for caution, particularly since persistence in tissues or the environment often creeps above expectations. Long-term research continues to chart unknowns, filling in data for future regulation and best practice.
Future Prospects
The pipeline of specialty amino acids rarely dries up, especially as researchers demand tools for ever-more-complex biology and materials science. 2,4-Difluoro-dl-phenylglycine anchors efforts to create hardier, more selective, and richly characterized bioactive molecules. Drug designers look toward new analogs, either shifting fluorine’s position or experimenting with other electron-withdrawing groups, hoping for even better metabolic and pharmacokinetic results. As computational chemistry becomes more predictive, synthesis routes get more efficient, and environmental testing lays groundwork for safe scale-up, expectation grows for broader adoption across pharmaceuticals, materials, and possibly agricultural biotech. The real progress comes from tight feedback loops between desk, bench, and clinic, with lessons from each field cycling forward to shape the next generation of molecular design.
Digging Into 2,4-Difluoro-dl-phenylglycine
2,4-Difluoro-dl-phenylglycine doesn’t show up at the local pharmacy or in an average high school chemistry class. It exists quietly in the background, driving progress in fields like pharmaceuticals and peptide chemistry. If you happen to work in a research lab or keep an eye on trends in drug development, you’ll know that molecules like these do the heavy lifting in early-stage synthesis long before pills make it to shelves. My time assisting science writers and talking with chemists taught me that the building blocks can be just as important as the final product.
Putting It To Work In Drug Development
Pharmaceutical companies have always chased unique building blocks to modify drug candidates. Small changes—like adding two fluorine atoms onto phenylglycine’s ring—can dramatically shift how a molecule acts in the body. 2,4-difluoro substitution helps researchers alter bioavailability, stability, and binding. Years ago, a medicinal chemist told me they spent months swapping different amino acids into drug designs, looking for one that would hang around long enough or dodge certain enzymes. It’s not glamorous work, but each tweak means new possibilities for treating disease.
This compound, with its specific structure, catches the eye particularly when researchers want to design protease inhibitors or peptidomimetics. A fluorine atom can stubbornly resist metabolic breakdown. When a glycine backbone is involved, it gives flexibility to the drug’s structure. Put these together as 2,4-Difluoro-dl-phenylglycine, and you’re looking at a tool that could help promising drugs last longer or fit better with a target protein.
Peptide Synthesis And New Horizons
The synthetic world is packed with options, but subtle differences in chemical structure change everything. Adding this molecule to a peptide can help create medicines that mimic nature but aren’t easily broken down by the body. As a result, researchers turn to 2,4-Difluoro-dl-phenylglycine when standard amino acids can’t get the job done. During work with a team producing enzyme-resistant peptides, I saw how tricky it could be to get new drugs approved. Each new building block has to clear rigorous scientific review, with a mountain of safety tests and data to back up every claim.
Safety, Research, And Supply Chain Issues
Any time a new chemical enters the drug pipeline, safety gets a front-row seat. Toxicity and environmental impact come under sharp scrutiny. 2,4-Difluoro-dl-phenylglycine doesn’t fly under the radar just because it’s a helper ingredient. Regulatory agencies demand thorough documentation—right down to the process that creates the first gram in the lab. From stories I’ve heard, pharmaceutical teams focus as much on sourcing high-quality precursors as on designing new drugs. Problems with inconsistent supply chains or contamination grind research to a halt.
Supply chain interruptions have been a growing concern, especially after recent world events. Producers and labs now look for reliable manufacturing partners, demanding audits and transparency. Having spent time discussing procurement with research managers, I know how quickly a promising project can derail over a bad batch of a specialty chemical.
Possible Solutions And Improvements
Science moves fast, but bottlenecks at any stage slow everything down. Producers of chemicals like 2,4-Difluoro-dl-phenylglycine can improve traceability and documentation, which helps not only with safety but also with efficiency. Greater investment in green chemistry could also cut environmental risks, something that matters for public trust and long-term sustainability. Collaboration between industry, academia, and regulatory bodies makes a difference, too. Past experience meeting with regulatory experts drove home how direct conversations between all sides make rules clear and progress faster.
As technology evolves, demand will keep shifting. 2,4-Difluoro-dl-phenylglycine’s story is just one part of the larger movement toward safer, smarter chemical innovation.
Breaking Down the Chemical Structure
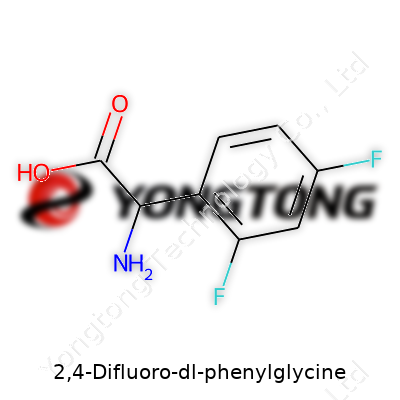
Walking into a chemistry lab, you’ll find 2,4-Difluoro-dl-phenylglycine on a shelf labeled with a formula: C8H7F2NO2. Digging deeper, the structure reveals a mix of elements seen in drug design and biochemical research. There’s a phenyl ring – that classic six-carbon aromatic circle – with not one, but two fluorine atoms attached at the 2 and 4 positions. These two little atoms make more difference than most people think, bringing new qualities to the molecule.
What gives this compound its personality is the glycine backbone. Glycine, the simplest amino acid, holds a special place in biology and medicine. Replace a couple of hydrogens on the phenyl ring with fluorines, and suddenly, you get 2,4-Difluoro-dl-phenylglycine. The nitrogen sneakily bonds into the central carbon, which also hooks up with the phenyl ring and a carboxylic acid group. In shorthand, you’ll see the structure written as: F-C6H3-CH(NH2)-COOH, with the “F”s at positions 2 and 4 on the ring. That fluorine isn’t just a spectator either. It’s got real impact on both reactivity and biological effect.
Why Fluorination Matters Here
Working in research, I’ve seen how small substituents on an aromatic ring make or break a compound. Fluorine atoms don’t show off with size, but they shake up electron density and boost metabolic stability. Add fluorines to an amino acid like this, and you can influence how the molecule interacts with enzymes. Many scientists use fluorinated amino acids to mimic natural ones, binding to enzyme sites with a little extra twist. It’s not just theory: drugs on the market often rely on a single fluorine for their punch.
Even outside pharmaceuticals, fluorination can slow down degradation, holding off enzymatic breakdown for longer periods. In the case of 2,4-Difluoro-dl-phenylglycine, that means researchers get to study processes in living cells for longer, test new pathways, and design better inhibitors for disease-related targets.
Applications and Concerns
Researchers value 2,4-Difluoro-dl-phenylglycine for peptide synthesis and enzyme inhibition studies. It stands out as a building block in creating molecules with predictable, targeted properties. Laboratories often incorporate this compound when looking to study enzyme interactions, fine-tune binding, or push into new territory in medicinal chemistry. That makes its purity, and structural accuracy, all the more critical. Contaminants or misidentified isomers quickly ruin experiments. I’ve personally tossed out batches of chemicals for this reason alone — sloppiness in synthesis or catalog labeling can claim weeks of work.
The dl- part signals a mixture of two mirror-image forms. In many biological processes, only one “hand” fits the lock. If both are present, results get muddied. For medicinal design, sorting out these enantiomers pays dividends. Investing in better separation methods — chromatographic techniques, crystallization, or even new synthetic routes — stands to benefit everyone from academic researchers to pharmaceutical developers.
Looking Ahead
With rising interest in smart drug design, these fluorinated building blocks deserve more study for stability, bioavailability, and target selectivity. Supporting open databases of accurate, peer-reviewed chemical structure data helps keep everyone honest and sharp. Even those with years of bench experience double-check reference spectra and melting points before committing resources or time.
Precision here isn’t just about the science, but about delivering reliable outcomes downstream — in research, medicine, and beyond.
The Realities of Dealing with Specialty Chemicals
2,4-Difluoro-dl-phenylglycine doesn’t turn up on every chemist’s bench, but those who do work with it know that routine doesn’t cut it. As someone who’s worked in small and large academic research labs, it’s clear that keeping it simple can mean the difference between a crisp reaction and a costly mess. No one enjoys the smell of decomposition or the unexpected fizz of a mishandled reagent. This compound demands a clear approach—treat it with respect, and the day runs smoother.
Keeping It Dry and Cool Matters More Than You Think
Every label says “store at room temperature, dry, tightly closed.” Folks get desensitized seeing that script on every bottle, but with 2,4-Difluoro-dl-phenylglycine you’ll want to pay attention. This material draws moisture like a magnet, so a humidity spike means trouble. At the university’s synthetic organics lab, leaving a bottle uncapped next to a running water bath caused more than one ruined experiment. Switch out the air for desiccant jars, keep caps tight, and stow the bottle on a low shelf far from heat. A high shelf, close to ceiling vents or lights, actually warms up more than room temp, risking degradation even if you can't spot it by eye.
Protecting the Compound, Protecting People
Experiments in my undergrad days taught me quickly: proper gear matters. Gloves, goggles, fitted lab coat, and closed-toed shoes stop surprises fast. You never want direct skin contact or particulates in your eyes—details the Material Safety Data Sheet repeats for good reason. If you’ve ever cleaned up after a dropped flask, splash exposure stings and lingers on the skin. For this chemical, the potential irritancy is enough to turn five minutes of care into a habit. Invest in a personal fume hood workspace if you’re opening multiple bottles or weighing out solids regularly, especially since powders drift with the lightest breath.
Labeling and Inventory: More Than Just Bureaucracy
Rare chemicals have a habit of getting lost at the back of the shelf. One winter, I found two expired vials, mismarked, and both set off my detector for minor vapors. Sharp, up-to-date labeling and clear logbooks stopped the problem the next year—easy systems build trust in every lab. That also means marking when you opened the bottle. If a batch is more than a year old, or if the powder has clumped or dulled, check quality before it goes anywhere near a reaction flask.
Minimizing Waste and Contamination
Practices from older chemists hold up well: always pour out a small quantity rather than dipping spatulas into the main stock. Cross-contamination destroys purity, and that costs more than the time to pour extra. Secure waste containers make a difference, and regular disposal prevents mysterious leftovers at the end of a project. Every waste drum left half-open or mislabeled raises risk for the whole team.
Keep Learning, Keep Improving
There’s no place for arrogance in chemical handling. I learned most from watching which techniques produced the best yields and cleanest workspaces. Talking to safety officers and more experienced chemists only sharpened my instincts. If anything feels out of place—caking, color change, unexpected odor—treat it as a problem to solve rather than a step to skip. 2,4-Difluoro-dl-phenylglycine deserves the same respect as any specialty reagent: treat it thoughtfully, and research runs better.
Purity isn’t Just a Sales Pitch
Every time someone talks about chemicals for research or industry, purity pops up as a term people throw around with weight. Working in a lab, you quickly recognize purity isn’t only about how “clean” a product looks. Especially when dealing with something like 2,4-Difluoro-dl-phenylglycine, you want to know what you’re actually getting before you even unbox the shipment. Sloppy purity can muddy experiments, waste money, and frustrate everyone who spent hours planning those syntheses or bioassays.
Life Behind the Labels: Purity Grades in Context
Selling chemicals is a competitive business. Suppliers compete on more than price—they’re catering to researchers, pharma teams, and chemical manufacturers, each with very different needs. Take 2,4-Difluoro-dl-phenylglycine. One supplier might offer what they call “analytical grade,” often with purity above 98%. These lots get snapped up by those running drug discovery experiments or HPLC verifications, where even tiny contaminants can send results off a cliff.
Technical or industrial users aren’t interested in paying premium prices if that extra percentage point of purity won’t affect their process. Some plants might use material at 95% purity, focusing on cost savings and stability over bleeding-edge accuracy. It’s a practical decision. For research, that few percent could mean the difference between a breakthrough and another confusing dead end. So, you’ll see options: one for people who need super-high purity, another for those who value productivity or cost control over lab-grade precision.
Who Decides What Grade Matters?
Not every application needs top-of-the-line purity, and making the wrong choice can be expensive. I’ve seen teams buy the highest available grade, thinking it’s the safest route, only to realize their work didn’t require that level of specificity. It’s not only about bragging rights to the “purest” sample; matching grade to use-case is practical science.
For 2,4-Difluoro-dl-phenylglycine, suppliers publish Certificates of Analysis, laying out the actual purity, heavy metal content, moisture, and other relevant tests. Reputable outfits document their quality control, so customers can match specifications to their application requirements. For highly regulated fields like pharmaceuticals, regulatory filings often require detailed traceability and confirmation of every compound’s source and grade.
Quality, Trust, and Real-World Experience
Experience in the lab teaches you to approach supplier claims with skepticism unless they’re willing to show real data. I've ordered from three separate vendors for amino acid building blocks in the same week and received wildly different results, even though documents claimed 98% purity across the board. Only after running my own NMR spectra did the “truth” about residual solvents or unknown impurities come out. This isn’t just academic: small differences can ruin an entire run or skew years’ worth of data.
So, the conversation about purity grades matters. If a chemical—especially something as nuanced as 2,4-Difluoro-dl-phenylglycine—is offered only in one grade, buyers should dig deeper or request custom purification. For anyone thinking about scaling up or introducing new compounds into sensitive processes, verifying more than a brochure, and forming open lines of dialogue with suppliers, pays off. Pushing for transparency, sharing analytical data, and focusing on true needs—those actions support safer science, better products, and real accountability.
Why Taking Chemical Safety Seriously Matters
Everyday work in chemical environments brings familiar sights: gloves stacked beside workbenches, goggles balanced on foreheads, lab coats used as shields. Some days, it’s easy to forget just how much hinge on ordinary habits. Take a compound like 2,4-Difluoro-dl-phenylglycine—one look at its structure and the warning flags fly. Experience working with fluorinated organics or aromatic amino acids offers a clear message: pay attention or pay the price.
Personal Protective Equipment: The Real Foundation
Getting too casual with your gear often leads to trouble. For this particular chemical, gloves stand as the first defense—choose nitrile or neoprene, not powdered latex. After swapping gloves too late once, I realized even sturdy options break down after extended contact with concentrated organics. Eye protection shouldn’t gather dust, either. I’ve seen a simple splash send someone straight to eyewash, and that short stumble to the station never feels short when vision’s at risk. Wraparound goggles or safety glasses rated for chemical use stop flying drops before they start a bigger problem. Lab coats and closed shoes finish the shell. Bare arms or sandals have no place when handling dusts or spills.
Ventilation Matters More Than You Think
Trusting a fume hood saves more than your lungs. 2,4-Difluoro-dl-phenylglycine doesn’t give off choking fumes like stronger acids or alkali solutions, but inhaling light powders wasn’t something I’d risk again after an accidental cough-up. As per safety data and regulatory guidance, keeping air clean means using a ducted chemical hood. Shaky “DIY” setups or open windows aren’t enough, especially during weighing or transfers. Fans circulate dust where you least want it, so venting air away tops the list.
Tidy Bench, Healthy Colleague
Messy setups breed accidents. Spilled chemicals blend, labels fall off, and the next shift inherits a headache. Learning from a few near-misses, cleaning as you go and clearly tagging containers helped keep things straight. Spatulas, weighing paper, and glassware shouldn’t share space. Once, a sprinkle of phenylglycine ended up next to an unrelated aldehyde—only sharp eyes and training prevented a possible reaction. Tidy, labeled stations protect everyone, not just the last one out the door.
Disposal and Storage: Small Steps with Lasting Impact
Disposing of this compound in the regular trash or down the drain isn’t just naive; it skips regulatory requirements that trace waste back to its owner. Separate hazardous waste bins make clean-up easier and prevent cross-contamination. Experience showed me labels fade quicker than memory; permanent markers and robust containers beat scribbled sticky notes. For storage, dryness and darkness limit breakdown and accidental exposure. Fire-proof cabinets or lockable chemical safes keep curious hands out, especially in shared spaces.
Training and Quick Response
Reading the data sheet doesn’t replace practice. Annual drills, spill kits, and knowing where to find the nearest eyewash station made our group quick to respond when it mattered. Sharing real-world incidents during meetings drove home lessons better than slides ever could. Reporting near-misses might feel awkward, but every honest account built a safer routine over time.
Smart Habits Outlast Warnings
Working with chemicals like 2,4-Difluoro-dl-phenylglycine rewards caution. I’ve seen how neglecting small steps snowballed into emergencies, but I’ve also watched good routines save the day. Most of what keeps us safe isn’t fancy equipment—it’s vigilance, teamwork, and learning from both mistakes and quiet days. Keep safety fresh, and let those best practices do the heavy lifting.