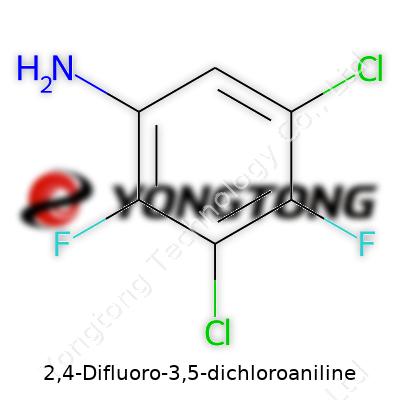
2,4-Difluoro-3,5-dichloroaniline: A Thorough Look Into Its Role and Relevance
Historical Development
Chemistry has a habit of circling back to small changes that unlock big possibilities. The story of 2,4-Difluoro-3,5-dichloroaniline lands right in that pattern. For decades, researchers have shaped aromatic amines to fit the needs of pharmaceuticals and crop protection. Adding fluorine and chlorine to traditional aniline didn't pop up overnight; it trails a path of trial and error by teams hunting for stronger, more reliable intermediates. Back in the late twentieth century, industrial labs and university benches watched halogenated anilines crop up as answers to persistent problems in chemical stability and reactivity. Fluorine brought the promise of resistance to metabolic breakdown, useful for turning molecules into lasting agents. Chlorine joined for its own quirks—tweaking polarity and sometimes bolstering antimicrobial traits. That's not a trivial tweak. As patent filings piled up and patents expired, demand for specialized anilines such as this one surged. Every step along the way, chemists looked for a mix of synthetic efficiency, safety, and performance, setting the stage for 2,4-Difluoro-3,5-dichloroaniline to move from academic curiosity to a regular fixture in industrial inventories.
Product Overview
2,4-Difluoro-3,5-dichloroaniline doesn't ride solo as some mysterious ingredient. Factories package it as an off-white to faintly yellow solid, usually with a distinct, sharp odor. It travels the world in drums or lined containers, flagged by distinctive labels because both chlorine and fluorine signal caution in handling. Its reputation as a key intermediate means it's less likely to show up in final retail products, but people relying on modern medicine or enhanced agricultural yields benefit from its indirect presence. Most people have never heard of it, but take a walk through any modern research facility or specialty manufacturer, and you'll find it on order lists.
Physical & Chemical Properties
With a molecular formula of C6H2Cl2F2NH2, this compound packs a punch. Every substitution changes its interaction with solvents, temperatures, and even sunlight. The melting point averages around 54–58°C, and it keeps a shelf-life if stored in cool, dry rooms away from direct light. It won't dissolve easily in water but takes nicely to organic solvents—think dichloromethane, acetonitrile, and similar hydrocarbon blends. This resistance to water keeps it stable in storage and use, sidestepping accidental hydrolysis and lowering the chance it'll escape into the environment. Both fluorine and chlorine bring high electronegativity, which makes the aniline ring less prone to random reactions while still keeping it open to well-designed chemical transformations.
Technical Specifications & Labeling
Industry standards call for tight purity—often pegged above 98% by HPLC, with specific limits on residual solvents or heavy metals. Color, melting range, and particle size count as everyday checkpoints. Every shipment wears labels with UN codes and hazard diamonds. Details about toxicity, emergency procedures, and safe ventilation are impossible to overlook. Manufacturers deliver all this data with the product, because the regulatory landscape won't excuse gaps. Experience taught me always to cross-check the label information and batch-specific certificates of analysis, not just for compliance but to avoid nasty surprises in downstream processes.
Preparation Method
Synthesis usually begins with a fluorinated and chlorinated nitrobenzene. Reductive amination, whether catalytic hydrogenation or chemical reducers like iron and acid, converts the nitro group to an amine. Control over reaction temperature, pH, and cleanup steps makes the difference between a clean yield and an expensive mess. Waste handling deserves real attention due to halogenated byproducts. Anyone working in process scale-up knows how tight tolerances for impurities must stay, lest the final product fail quality checks.
Chemical Reactions & Modifications
The compound’s substituted ring can be a springboard for modifications. Chemists use its halogens to guide coupling reactions—Suzuki, Buchwald-Hartwig, or other cross-couplings. In these pathways, boronic acids or palladium catalysts inch close, using the electron-withdrawing nature of fluorine and chlorine to craft custom molecules for diverse applications. The amine transforms to amides, ureas, or carbamates for further use. I’ve seen these modifications spark the development of highly potent herbicides and refined drug scaffolds. The speed of these reactions and the range of ensuing products keep researchers motivated and suppliers in business.
Synonyms & Product Names
Trade and scientific circles recognize this molecule under several aliases: 2,4-Difluoro-3,5-dichlorobenzenamine, DFDC aniline, and sometimes as specific code names in proprietary chemical catalogs. Differences in labeling spring up between vendors and regions, but CAS numbers function as the true universal ID. Mislabeling or confusion in purchase orders can stall a project—one of those small details that creates headaches if not tracked closely in inventory systems.
Safety & Operational Standards
Safety teams don’t take this compound lightly. Gloves, goggles, and laboratory coats become standard. Ventilated hoods and spill kits wait nearby. Chlorinated and fluorinated anilines may cause irritation or worse if handled carelessly; documented instances of skin and respiratory reactions have prompted strict workplace exposure limits. European regulations and US OSHA standards treat the compound with seriousness, flagging it for toxicity to aquatic environments as well. Every chemist develops a healthy respect for these compounds after one or two minor incidents, and larger companies log incidents to keep teams aware of the hazards.
Application Area
Pharmaceuticals, crop protection, and specialty polymers count as the main arenas. In pharma, it’s a trusted scaffold for building up enzyme inhibitors, antineoplastic agents, or CNS-active molecules. The pesticide world demands robust intermediates for weed and fungi management, with chlorine and fluorine conferring both environmental-persistence and selectivity against pests. Specialty materials producers carve out niches in pigment and electronic chemical supply chains, where small tweaks in molecular design change colorfastness, conductivity, or other key parameters. Seeing such versatility makes the compound valuable for process chemists racing to devise new, IP-protected molecules.
Research & Development
Each year, fresh research re-examines both the core structure and downstream derivatives of 2,4-Difluoro-3,5-dichloroaniline. Scientists hunt for green synthesis methods, reduce halogenated waste, and scout replacement routes that cut costs without sacrificing quality. Computational chemists simulate its behavior in metabolism or material science settings, helping design safer or more effective analogs. The pace of academic publications and patent filings shows no sign of slowing, signaling that this compound continues to shape frontline innovation.
Toxicity Research
Animal studies and computational models both point to acute toxicity, giving rise to clearly defined handling and disposal rules, not just idle warning labels. Water solubility remains low, so its risk comes from accidental release or poor incineration practices rather than leaching into groundwater. Chronic exposure data remains thinner than for classic aromatic amines, but enough evidence links it to skin and respiratory distress to limit worker exposure. Toxicologists are still unraveling long-term environmental impact, which means any manufacturer ignoring disposal best practices risks regulatory backlash and community pushback.
Future Prospects
Demand for halogenated intermediates like this one only ratchets upward in a global economy hungry for improved pharmaceuticals and efficient agrochemicals. Specialty chemical manufacturers chase greener, more sustainable synthesis—less hazardous solvents, zero-waste strategies, and renewable feedstocks stand as practical goals. Digital simulation speeds up molecule design, but human intuition and skill still drive most of the creativity behind molecular tweaking. If new toxicological concerns surface, regulatory adjustments could render long-standing methods obsolete almost overnight. On the flip side, advances in environmental remediation and monitoring may keep doors open for compounds offering genuine benefit. Keeping ethics, worker health, and environmental stability in balance marks the true challenge. Those invested in this field watch the shifting regulatory tides and scientific advances, betting on both responsible stewardship and technical leaps forward.
Breaking Down the Name
Many people see a name like 2,4-Difluoro-3,5-dichloroaniline and feel lost before they even begin. I remember learning organic chemistry and feeling like I was translating a foreign language. Over time, what helped was piecing apart those intimidating labels and connecting them to real atomic relationships. Smelling the sharp scent of anilines in lab stays with you—and so does the realization that every substituent tells you where atoms like chlorine or fluorine lock into place on a benzene core.
Where the Formula Comes From
The heart of any aniline sits in a benzene ring. If you add an "aniline" part, you have an NH2 (amino) group joined to the ring. In this specific chemical, the amine lands at the number 1 position. Scientists number the carbons around the ring to map where extra atoms attach. Chemistry doesn’t reward guesswork—accuracy guides names.
This name shows two fluorines at the 2 and 4 positions, plus two chlorines at the 3 and 5 positions. Plugging these groups onto the benzene ring, you get a structure that’s fuller than most: two carbons swapped for chlorine, two for fluorine, one joined to the amine.
The Formula Unveiled
I still remember sorting through textbooks, cross-referencing models, and drawing out ring structures with different colored pens. If you lay it out, you see that for each halogen—fluorine and chlorine—one hydrogen leaves. So, starting from C6H5NH2 (plain aniline), knocking off four hydrogens due to the four new substituents, and adding their atoms, you arrive at the right numbers.
For 2,4-Difluoro-3,5-dichloroaniline, the chemical formula reads:
- C6H2Cl2F2NH2
Or, reorganized for clarity: C6H3Cl2F2N (since NH2 becomes “N” and three hydrogens remain attached).
Why Details Matter in Chemical Formulas
Missing a detail with chemical formulas often spells trouble in the lab—one wrong atom and compounds lose their intended purpose. At a pharmaceutical firm I worked with, a misread drawing created a batch of useless product, costing thousands and generating extra waste. Knowing which atom sits where goes directly to safe handling and correct function, whether crafting a crop-protection agent, researching new antibiotics, or exploring dyes and pigments.
Chemicals like 2,4-Difluoro-3,5-dichloroaniline can seem crafty because, even with a straightforward backbone, properties swing wildly with a small change. Fluorine and chlorine aren’t just for flavor on packaging; they shift reactivity, toxicity, and how molecules act in the real world. One small difference in the ring makes the gap between a treatment and a toxin. For anyone working with organic chemicals, this illustrates that reading and understanding formulas means more than homework—it guards quality, workplace safety, and public health.
Getting it Right and Moving Forward
Exactness in handling names, formulas, and the knowledge behind them tunes chemistry for invention and responsibility. Whether you’re in a lab, classroom, or looking up ingredients for research, a sharp eye for structure isn’t optional. We stay safer and get better results by locking these connections into our memory, making sure one glance at a name can map out an entire molecule in your mind.
Why This Chemical Matters
Anyone who’s ever followed the journey of a product from raw material to finished good has seen how often chemicals work behind the scenes. 2,4-Difluoro-3,5-dichloroaniline fits in this story, mostly because it plays a key role where science and global industry meet. This compound, with both fluorine and chlorine atoms attached to an aniline base, goes beyond laboratory settings. Farmers, pharmaceutical developers, and even those in dye manufacturing all draw value from it, often without hearing its full name tossed about.
A Backbone in Crop Protection
Agriculture leans on chemistry, especially in recent decades. 2,4-Difluoro-3,5-dichloroaniline appears in the synthesis of several important crop protection agents. Production of certain herbicides, fungicides, and insecticides would stall out without it. Take the example of specific triazole fungicides—chemical building blocks like this one become essential for creating molecules that can fight plant diseases without harming crops. As farms push for greater yields from less land, tools for pest control mean fewer losses, steadier food prices, and improvements in global food security. That’s what I see having grown up seeing fields alternately thrive or fail depending on pest pressure and weather swings.
Sparking Advances in Pharmaceuticals
Any new prescription on the market goes through years of development. Chemists shaping potential treatments for cancer, infections, or neurological conditions sometimes find their answer starts with aniline derivatives. The unique combination of atoms in 2,4-Difluoro-3,5-dichloroaniline allows medicinal chemists to attach new groups, test ideas quickly, and dial in properties like solubility or metabolic stability. Researchers cite its use in lead optimization, particularly for drugs targeting enzymes where selectivity and reduced side effects matter. Choosing the right building blocks means patient therapies reach the finish line safer and faster. A friend in pharmaceutical process research once compared it to choosing the right canvas for a masterpiece—it sets everything in motion from the beginning.
Color and Specialty Material Manufacturing
This chemical steps up in dye manufacturing too. The textile world demands colors that don’t fade or wash out. Adding fluorine and chlorine gives molecules the muscle to hang on in sunlight or with repeated washing—something I noticed after seeing my favorite shirt still vibrant years later. 2,4-Difluoro-3,5-dichloroaniline forms part of the chain for these dependable dyes. Manufacturers of specialty polymers and coatings use it for similar reasons, seeking resistance against heat and chemicals. Whether coating electronics or assembling sturdy materials for transportation, the durability starts at this fundamental level.
Tackling Safety and Sustainability
Every chemical story comes with questions—what about worker exposure, waste handling, or long-term effects on the environment? Stringent regulations exist because compounds with halogen atoms like chlorine and fluorine sometimes resist breaking down. Companies and governments push for best practices: sealed systems, ongoing monitoring of air and water, investment in greener chemistry, and building safer analogs for the future. Finding better pathways means involving everyone from factory floor workers to environmental analysts, balancing new technology with responsibility.
Room to Grow
Chemistry moves quickly, and so do the rules of the market and regulation. New uses could arise in electronics or even medical imaging as research expands. For now, watching where 2,4-Difluoro-3,5-dichloroaniline lands—be it on a crop, in a medicine, or on a fabric—reminds us how deeply chemical building blocks shape industries and daily life.
Understanding the Chemical
2,4-Difluoro-3,5-dichloroaniline shows up in many labs that focus on advanced material research, pharmaceuticals, and crop protection chemistry. Its value comes hand-in-hand with its hazards, which means giving it the respect it demands—storing and moving it with care, not letting convenience drive decisions. Even though this compound is only one small part of a larger synthesis, poor handling can turn it into a disaster, both for safety and for quality control.
Safe Storage Practices
This chemical doesn’t play well with heat, sunlight, or moisture. Direct sun, wavering room temperatures, or a leaky roof spell trouble here. Tucking it away in a cool, dry spot—think below 25°C, out of the sun’s path, away from water sources—keeps its chemical stability in check. Nobody wants to pull out a container a month later to find clumped or decomposed material.
A bench or shelf in a busy lab won’t cut it. You want a secure, chemical storage cabinet, ideally outfitted for corrosives, to lock in fumes and keep others safe. At my old university lab, we saw firsthand how easy it is for vapors to seep into open shelves. All it took was one careless cap left open, and suddenly the whole room picked up a biting, chlorinated smell. Corrosive cabinets with proper ventilation solve that problem before it starts.
Clear labeling beats guessing anytime. Handwriting rubs off, generic labels fade, and it’s not hard to mix up bottles during a long day. A proper hazard label, product name, and date make sure anyone can recognize the chemical and its storage rules at a glance. Safety comes from the basics: unmistakable bottles, no mystery containers, and current inventory lists to keep tabs on quantities and shelf life.
Direct Handling and Personal Safety
Gloves, goggles, and a sturdy lab coat form a line of defense against accidental skin or eye contact. Even a small spill can burn or irritate, and solvents used with this compound often kick up dangerous fumes. In practice, a fume hood should always be in play for any weighing, mixing, or transfer work. I remember one project where a colleague bypassed the fume hood “just this once.” The lingering smell told the rest of us, and we shut down operations while air handlers did their job. No experiment is worth that risk.
This is not something for open benches or quick “splash and dash” transfers. Clean, closed systems, dedicated spatulas, and sealed waste containers keep things safe. You gain peace of mind by never mixing this compound near incompatible chemicals, especially oxidizers or strong acids. Incidents often trace back to poor chemical compatibility checks, so a laminated compatibility chart pairs well with your storage cabinet.
Spill and Waste Management
Even if you do everything right, accidents happen. A spill kit stocked with absorbent pads and neutralizing agents belongs within arm’s reach, not locked away down the hall. Trained staff move confidently, contain spills, and dispose of waste using protocols that fit both regulations and local disposal rules. I’ve watched as novice researchers froze during a spill, not knowing step one. Regular drills help. Waste builds up too—hazardous waste labels and proper containment keep everyone clear of unnecessary exposure. Local environmental authorities often audit these disposal streams, so shortcutting here just piles up long-term headaches.
Room for Improvement
Not every research site can afford top-tier storage cabinets, but basic steps—controlled temperatures, dry and well-ventilated storage, proper labeling—give returns well above their modest cost. Audits and training sessions, even just once a year, reinforce these habits. In all my time working with specialty chemicals, it’s always the crew with the clean storage room and the sharpest labels that avoid mishaps. Some investments, like a fume hood or a drum with a lockable lid, pay for themselves the first time someone gets distracted on a busy day. No one wins when safety takes a back seat to speed or cost-cutting.
Looking Closer at Chemical Risks
A lot of specialty chemicals out there don’t come with clear warning labels, but that doesn’t mean they’re safe to handle without thought. 2,4-Difluoro-3,5-dichloroaniline is one of those names you rarely hear outside a lab or chemical plant, yet it pops up in research journals and shipment manifests for good reason. This compound sits in a class of anilines often used to make other chemicals, paints, or crop protection agents.
Anyone who’s worked with aromatic amines knows these materials aren’t like table salt or diluted vinegar. Scientists learned a long time ago that many substituted anilines affect living things—even in small doses. Organic chemists flagged this pattern after early industrial accidents led to skin burns, eye irritation, or more subtle health issues down the road. Some aniline derivatives are linked to problems in the liver, or in worst cases, changes to blood that leave people weak and sick. I’ve seen old lab safety manuals with warnings about wearing gloves, goggles, and using chemical fume hoods just for weighing out a few grams of these compounds.
Acute and Chronic Health Concerns
While there isn’t a mountain of public data for every compound, we can draw a line between 2,4-Difluoro-3,5-dichloroaniline and others in its group. Chlorinated and fluorinated anilines tend to cause more skin and eye damage than their plain cousins. Some, if inhaled or touched, spark strong irritation. Add those fluorine and chlorine atoms, and toxicity often steps up. These atoms aren’t just decorative—the body can’t always break them down fast, so they linger, causing more harm than simple molecules.
Labs handling this chemical usually mark it as hazardous and require spill kits, gloves, and goggles for a reason. Even if you don’t work in a research lab, environmental persistence should worry everyone. What ends up in water or soil doesn’t vanish overnight. Researchers document how small amounts of these chemicals move through water supply systems, potentially entering crops or wild fish. Stories from countries with loose chemical regulations remind me what happens without proper care: Someone pours waste down the drain, and years later, children play in streams with residue nobody can even see.
Regulation & Better Practice
Regulators already track chemicals like 2,4-Difluoro-3,5-dichloroaniline. European REACH protocols, for example, ask manufacturers to register toxicity data. The US EPA’s chemical database lists chemicals in this group for toxicological concern. That doesn’t always mean companies provide clear safety advice, though. I’ve met engineers who still rely on old habits or hope for the best. Reading the safety data sheet, using double nitrile gloves, and keeping reaction vessels covered isn’t overkill—it’s just self-preservation.
Solutions exist beyond technical compliance. Companies can invest in safer substitutes without halogens. Chemists learn to design greener chemicals as part of their training now. On the ground, every workplace can run training sessions showing what exposure really means. Connecting these risks to real people’s experiences—showing the results of chemical burns, or how chronic exposure disables workers—drives safer behavior far better than bold-letter warnings.
Ultimately, 2,4-Difluoro-3,5-dichloroaniline deserves respect for the risks it brings. It’s less about the rare accident and more about how unnoticed, small exposures add up when folks let down their guard. Safety isn’t just a checklist; it’s the culture that keeps people at work and healthy at home.
Connecting Chemicals to Real Life
2,4-Difluoro-3,5-dichloroaniline has its own fingerprint in the chemical world: 3939-09-1. Each compound gets a CAS number, a unique tag that helps chemists, pharmacists, manufacturers, and regulators cut through the confusion of complex naming systems. Simple as it sounds, this number saves people from costly mix-ups in labs and warehouses, and keeps health and safety on solid ground.
Looking Beyond the Number
Why should anyone, aside from a chemist, care about a mouthful like 2,4-Difluoro-3,5-dichloroaniline? In my years of chasing raw material shipments for paint formulas, that sort of name meant more than a line on a purchase order: it meant the glue holding together entire processes. Miss a number, and an order can go sideways. Tracking down a compound using the wrong name could halt a production line or, worse, invite dangerous substitutions.
The chemical industry leans on details, and small errors scale up quickly. When the wrong chemical lands in the wrong place—think pesticides, pharmaceuticals, or cosmetics—the results aren’t just embarrassing. They pose risks to workers, consumers, and the natural world. Regulators from the EPA, OSHA, and their counterparts overseas demand clarity. The CAS number delivers just that.
Seeing the Big Picture
Mistaking a chemical’s identity in the digital age can spark bigger problems. With global trade pulling supplies from several countries, batches shift hands fast. Many countries use different names, but the CAS number never changes. In traceability work—especially after a supply chain recall or safety scare—pulling up the right number makes all the difference. You can avoid scandals, lawsuits, or dangerous recalls with a bit of attention up front.
The Power of Consistency
Chemicals like 2,4-Difluoro-3,5-dichloroaniline go into everything from specialized coatings to research reagents. During my stint working with regulatory data, I saw firsthand that a simple database built around recognized CAS numbers could clean up a web of ambiguity. That cleaned-up data keeps inventory audits honest, improves emergency response, and helps meet international shipping guidelines—something the industry can’t afford to mess up.
Building Trust from the Ground Up
People rely on the chemical industry for safe medicines, pure water, clean air, and sustainable products. Each unique identifier in the form of a CAS number works a bit like a safety lock. You don’t need a PhD to see why trust in the industry starts with good record-keeping. Having the right CAS number—3939-09-1 for 2,4-Difluoro-3,5-dichloroaniline—connects researchers, workers, and users with the facts they need.
Facing Tomorrow’s Challenges
Global sourcing continues to stretch logistics systems, challenging how quickly and safely chemicals move across borders. As the world pushes toward greener processes and stronger consumer protections, simple identifiers like CAS numbers will play an even stronger role. Tech upgrades in barcoding and data sharing can smooth out record mismatches, keeping products honest and people safe. Decades working alongside technical staff taught me: numbers don’t just fill boxes, they close loops and protect communities.