2',4'-Dichloroacetophenone: A Commentary on Its Legacy, Use, and Future
Historical Development
Modern chemistry owes much to the story of 2',4'-Dichloroacetophenone. People started looking at acetophenone derivatives for both industrial and defensive purposes early in the last century. The chlorination of acetophenone produced compounds like this one that caught attention during wartime research and industrial expansion. History ties this compound to riot control agents and research in organic chemistry, where folks experimented to find new uses beyond military settings. Each generation of chemists played a role in tracing its behavior and pushing its limits in labs and practical applications.
Product Overview
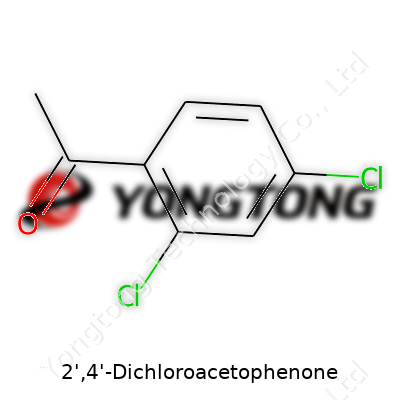
You’re looking at a compound with a clear chemical structure: acetophenone with two chlorine atoms at the 2' and 4' positions on the benzene ring. Chemically, this changes more than just the molecule’s address. The dual chlorination impacts how researchers and manufacturers use the compound. Factories produce it in crystalline or powdered form; it usually comes off as a pale yellow solid with a distinct, pungent odor that leaves a mark if you bump into it. Large chemical suppliers market it under various trade names, but the backbone stays the same.
Physical & Chemical Properties
Diving into its profile, 2',4'-Dichloroacetophenone holds a melting point around 54-56°C, boiling at about 283°C under atmospheric pressure. The molecule rings in at a molecular weight of 203.06 g/mol. Like other halogenated aromatics, it resists water and favors organic solvents like ether, chloroform, and ethanol. Stability is its game; the chlorines not only harden it against quick reactions but also make it persistent—something labs both appreciate and contend with during waste handling. Its volatility demands careful storage and measured handling, especially at scale.
Technical Specifications & Labeling
Labels on commercial packages spell out hazards in line with international chemical safety frameworks. You’ll see CAS number 2234-16-4 printed beside pictograms signaling acute toxicity and irritation risk. Suppliers usually provide purity between 98% and 99%, along with clear statements of moisture content and impurity profile. Batch certificates ensure industry and lab customers know exactly what they’re getting, and MSDS sheets hammer home the need for gloves, goggles, and ventilation whenever someone’s in contact with the material.
Preparation Method
The standard preparation steps into Friedel–Crafts acylation territory. Here’s how it plays out in most setups: You start with 2,4-dichlorobenzoyl chloride, react it with acetophenone in the presence of a Lewis acid catalyst such as aluminum chloride. Reaction conditions get adjusted for optimal yield and purity, sometimes involving purification by recrystallization or distillation under reduced pressure. Experienced chemists monitor exothermicity and fumes, underscoring the need for control throughout the process.
Chemical Reactions & Modifications
This compound’s ketone group sits wide open for further tinkering. Reductions can pop out corresponding alcohols, or folks can run condensations, especially in the hands of synthetic chemists chasing new molecules for pharmaceutical research. The aryl chlorides make for prime Suzuki and Heck coupling partners, opening doorways to build up more complex aromatic architectures. Modifications usually require strong bases or nucleophiles—conditions where lesser compounds might break apart, but the backbone here flexes without shattering.
Synonyms & Product Names
The world of chemicals stays messy with naming conventions, and 2',4'-Dichloroacetophenone wears a few hats. Some call it CN (not to be confused with cyanogen compounds), others refer to its systematic name as 1-(2,4-dichlorophenyl)ethan-1-one. Trade names fill out the rest, depending on regional suppliers and application sectors. Each label reminds people to treat it with the respect strong organics demand.
Safety & Operational Standards
You don’t forget the first time a whiff of CN hits your nose. Irritation comes fast—eyes water, throat tightens, and skin tingles after contact. Safety guidelines stress enclosed handling systems, robust glove selection, and real-time air monitoring. In storage, chemical-resistant containers tucked into cool, ventilated rooms lower risks. Regulations tie into its history, classifying the compound as a controlled substance for some applications, especially where potential for misuse exists. Environmental teams require detailed waste management plans—incineration with complete containment wins, avoiding any accidental release into water streams.
Application Area
Beyond its headline-grabbing role as a tear gas agent in law enforcement, industrial chemists reach for 2',4'-Dichloroacetophenone as a building block in organic syntheses. The dual chloro structure serves the needs of agrochemical manufacturers designing new pesticides. Pharmaceutical research sometimes creates related structures to probe enzyme behavior. Some specialty labs pursue its derivatives for dyes and advanced functional materials, though scale stays limited by safety concerns and regulations.
Research & Development
Academic groups keep poking at the frontiers with this molecule. Studies look for ways to achieve safer, greener synthesis methods—less reliance on aggressive acids and chlorinated reagents. Others test new neutralization methods to counteract its effects in field situations. Analytical chemists work up better detection methods for environmental monitoring. Industrial research seeks to harness its reactive groups for new catalytic cycles or materials science breakthroughs, showing the molecule’s adaptability despite a reputation built on riot control.
Toxicity Research
Toxicologists track the effects after exposure down to molecular pathways. Skin, eye, and lung irritation dominates acute cases. Studies from animal models suggest possible chronic hazards, with organ damage stemming from repeated contact or inhalation. Epidemiological data from occupational settings show increased respiratory symptoms and impact on mucous membranes. Regulators use this body of work to shape exposure limits and first aid protocols, guiding industries on minimum safe practices. Protective gear, engineering controls, and medical surveillance act as frontline defenses for workers.
Future Prospects
People now look beyond the legacy of 2',4'-Dichloroacetophenone. Environmental questions spark efforts to limit emissions and replace harsh reagents in its production. Green chemistry initiatives hope to phase in alternative synthesis paths, lowering waste and risk. Solid-state chemists see a future in functionalized derivatives, potentially contributing to electronics or specialty coatings. The regulatory climate pushes for continual safety review, calling on manufacturers to innovate rather than stick with old playbooks. The trajectory traces a compound with a controversial past toward a future ruled by responsibility, scientific curiosity, and a demand for practical results that do not come at the cost of health or the environment.
Understanding the Compound
2',4'-Dichloroacetophenone falls under the group of chlorinated aromatic ketones. Working in chemistry labs during my graduate years, I've seen this compound pop up on reagent shelves enough to respect its niche. Its chemical structure—two chlorine atoms lodged on a phenyl ring tethered to an acetyl group—might seem simple, but this backbone opens the door to a batch of specialized uses.
Industrial Uses that Stand Out
Factories and chemical manufacturers don’t just keep 2',4'-Dichloroacetophenone around for display. Among the most common uses, this compound finds a role as an intermediate during the synthesis of complex chemicals. Think about dye and pigment manufacturing: the presence of dichloro groups makes it reactive, which factories harness to add stability or unique properties to colorants. Small changes in a molecule can spell the difference between a fabric dye fading in weeks or surviving a hundred cycles in a washing machine.
In pharmaceutical work, things get interesting. Medicinal chemists use 2',4'-Dichloroacetophenone to introduce diversity into their molecule libraries. It's a step along the road to new painkillers, anti-fungal agents, and other drugs. The compound's chlorinated pattern helps scientists mimic or tweak natural substances, hoping to create something with medical value. More than a reagent, it’s part of the experimentation that pushes medicine forward.
Application in Tear Gas Formulations
My own uncle, a retired police officer, shared stories of training with tear gas in the 1980s. Many law enforcement and military supplies relied on chemicals much like 2',4'-Dichloroacetophenone. In fact, it figured prominently as the active ingredient in certain irritant gases, sometimes coded as CN gas. These canisters appear in riot control, granting crowd dispersal without permanent harm. Of course, modern perspectives question the ethics and safety of these agents, with some countries dropping these chemicals in favor of new formulas. There’s also tighter regulation—after reports of toxic effects, many agencies restrict or outright ban the compound’s use in crowd control.
Research and Analytical Work
In the lab, researchers use 2',4'-Dichloroacetophenone as a reference compound or chemical building block. It’s a tool for those charting unknown paths in organic chemistry. By reacting it with other chemicals, teams can map out reaction mechanisms or create substances for analysis—vital for quality control in drug and textile factories. Its predictable reactivity helps save time and reduce wasted effort, which any seasoned chemist appreciates.
Problems and Safer Handling
Potential hazards come with this compound. Exposure, especially in the absence of proper ventilation, can irritate eyes and respiratory systems. Long-term mishandling risks severe health effects. As a solution, industrial safety guidelines set limits on airborne concentrations, and public health experts advocate thorough training for anyone handling or disposing of it. Labs stock personal protective gear—goggles, gloves, lab coats—real staples for keeping technicians safe.
Environmental Impact and Future Directions
Interest in green chemistry keeps growing in response to environmental concerns. Processes that depend on chlorinated organics like 2',4'-Dichloroacetophenone face new scrutiny. Companies fund research into less toxic alternatives, aiming for similar performance with a lighter ecological footprint. Waste management technologies scale up to filter and neutralize hazardous byproducts before they reach waterways. Ongoing scientific innovation looks for routes that lower risk, making workplaces and neighborhoods more secure.
Understanding the Risks
2',4'-Dichloroacetophenone, often called CN gas, pops up in some law enforcement tools and industrial labs. The stuff carries more risk than most chemicals you’ll run into while handling routine lab work. Breathing in the vapors or getting the dust on skin brings eye and throat irritation, shortness of breath, and strong pain. I’ve watched firsthand what happens if someone underestimates it—burning eyes, hacking cough, and panic spread through a lab much faster than you think possible. Stricter attention to detail in handling CN makes a big difference, not only for safety but for peace of mind.
Protecting Your Skin and Lungs
Work only in spaces with reliable, well-maintained fume hoods. One broken blower or cracked sash, and fumes build up before anyone gets a warning. Keeping the door closed just isn’t enough with CN; the fumes search out every open pore and drifting current. Trusting goggles, thick gloves (nitrile, not latex), and fully-buttoned lab coats every time pays off. A basic mask can’t block the vapors. Respirators with filters rated for organic vapors (and kept in working shape) step in where lesser equipment falls short. Take the extra minute to double-check seals and fit—your air supply matters far more than tidy lab benches.
What To Do If Something Goes Wrong
Even in labs with routines nailed down, mistakes pop up. Spills, unexpected fumes, or messy glove changes lead to trouble. Flood eyes and skin fast with generous water—don’t reach for fancy solutions or wipes, just use the nearest eyewash or shower. Supervisors should post emergency numbers where everyone can find them. Move into fresh air immediately if vapors fill your space. Do not assume that fanning the air or opening a window fixes the problem—leave until the trained response team clears the area. Tell doctors exactly what you were handling so they don’t lose precious time guessing. I once watched a quick, confident response stop a small incident from turning serious. Preparation builds strong habits.
Safe Storage and Transport
Containers for CN must seal tightly, no exceptions. Stash them in chemical safety cabinets marked for toxins. Temperature swings and sunlight weaken packaging, risk leaks and create hazards you can’t see with the naked eye. Never use regular fridges or generic shelves—even a few degrees of heat change can release enough vapor to fill a room. When moving bottles from storage to bench, double-bagging and spill trays lower your risk from sudden drops or bumps in tight spaces. Label every bottle clearly. Don’t trust your memory to keep you safe.
Handling Waste and Cleanup
Disposal brings its own set of problems. Pouring CN down the drain sets up your community and co-workers for years of trouble. Secure waste containers and work with a certified hazardous waste team. I’ve found that regular waste audits and refresher workshops catch bad habits before they stick. Everyone who handles a mop, wipe, or squeegee needs clear rules: every item used in cleanup goes into the sealed waste, no shortcuts allowed. Testing wipes and spilled material for contamination saves time and money down the line.
Training and Culture Matter
Sharpening everyone’s skills and memory about chemical dangers shapes safer labs and workplaces. Regular training, practice drills, and honest feedback make the biggest difference. CN gas doesn’t forgive shortcuts. Leaders who take safety seriously never have to remind folks why standards matter—people remember what’s at risk. Drawing on my own experience, I see that real safety culture grows from people who hold each other accountable, ask questions, and take pride in clean, orderly work.
Molecular Formula: Getting to the Roots
2',4'-Dichloroacetophenone belongs to the family of acetophenones, but the “dichloro” part immediately stands out. Here, two chlorine atoms attach themselves to the aromatic ring, making it a chlorinated derivative. The molecular formula comes out to C8H6Cl2O. Eight carbon atoms, six hydrogens, two cholrines, and one oxygen—it seems simple, but every atom in this setup has purpose.
Visualizing the Structure
Let’s picture the core: a benzene ring, which is a hexagon arrangement of six carbons with attached hydrogens. The acetophenone backbone starts out as a benzene ring with an acetyl group (COCH3) stuck onto it. For 2',4'-dichloroacetophenone, two hydrogens on the benzene get swapped out for chlorine atoms.
If you count carbons starting from the acetyl group as position 1, and circle around the ring, chlorine atoms sit at positions 2 and 4 (or more formally, ortho and para to the acetyl group). The rest of the ring carries hydrogens. So, the skeleton looks like this: a benzene ring, an acetyl group, chlorines at 2 and 4, and hydrogens in the remaining positions. That’s the map of this molecule.
Why Structure Matters in Chemistry, Medicine, and Beyond
A molecule’s shape and makeup isn’t just an academic detail. Researchers and chemists stare hard at these structures because tiny tweaks change how a molecule behaves. In this case, the dichloro groups don’t just add a bit of weight—they crank up the reactivity and, crucially, toxicity.
2',4'-Dichloroacetophenone has seen real-world use as a tear gas (often called CN). Back in college labs, we saw how even mild exposure to traces caused instant irritation. The positioning of the chlorine atoms (precisely at 2 and 4) sets this molecule apart from others in its family, heightening both potency and volatility. That brings up safety, ethics, and handling processes that nobody in the chemical industry overlooks.
Learning from How Chemical Structure Drives Policy and Practice
Over the last decade, tighter regulations have kicked in around substances like 2',4'-dichloroacetophenone. Regulatory agencies examine not just what a chemical does but why it does it, right down to molecular architecture. When risk or benefit rides on those details, we can’t afford shortcuts. Handling, storage, personal protection—all these standards connect straight back to the molecular structure. That knowledge saves real lives.
Chemists and educators often rely on up-to-date research, peer reviews, and direct experimentation to confirm chemical properties. The published data on 2',4'-dichloroacetophenone leaves no question: its structure shapes its interactions with airways, skin, and eyes—which is why governments monitor every gram.
Planning for Safety and Responsible Use
Tackling the risks tied to chemicals like this one means focusing on clear labeling, rigorous training, and solid supply chain traceability. Institutions that use 2',4'-dichloroacetophenone in research or manufacturing take their cues from leading scientific bodies and real-world incident data. Wearing the right PPE and working with proper ventilation aren’t optional extra—they’re built into professional practice because the molecule’s structure demands respect.
If we keep asking questions, sharing new findings, and taking daily precautions informed by hard evidence, we make sure useful compounds don’t turn into preventable hazards. Behind every structural detail lies a set of choices about safety, society, and science working together.
Getting Serious About Safety
2',4'-Dichloroacetophenone isn’t a household name, but those who’ve worked in labs know this chemical as more than just a tongue-twister. Used mainly in research or legacy riot-control projects, it’s enough to know it comes with real risks. Mishandling invites trouble, and the stakes reach beyond workplace guidelines. In my own line of work, it’s easy to see how one careless storage decision can threaten everyone’s well-being, not just on paper but in the lived, breathing world of the lab or storeroom.
Understanding the Chemical’s Nature
Ask any chemist to describe this compound and you’ll hear about volatility, harsh odor, and how it behaves around heat and light. It’s an irritant—get some on your skin or in your eyes and it burns. Leave it uncapped and you’ll catch the vapor. Forget about temperature control or ignore moisture, and the risk ramps up. Take these as signals, not suggestions.
Basics of Responsible Storage
Every responsible person starts with basics. Glass bottles with tightly fitting lids, not plastic containers or improvised jars, stand as the standard. Cool and dry conditions set the tone—storage near radiators or under sunlit shelves invites trouble. I’ve seen old storage closets where the air felt damp and the labels peeled. That’s not the scene for this chemical. Stash it away from chemicals that react with organics or acids, and keep it from oxidizing agents. You don’t want to create an invisible danger zone by accident or convenience.
Good ventilation in storage areas acts like an insurance policy—you hope you won’t need it, but you’ll be glad it’s there if a leak ever happens. Label everything clearly. I’ve walked into rooms where faded labels bred confusion, and that’s how mistakes creep in. If there’s a spill, a fast response with the right absorbents keeps the mess from spreading, but a well-run space means fewer emergencies from the start.
Personal Experience in the Lab
Back in graduate school, a friend once forgot to tighten the lid on a bottle of a similar compound. By the next morning, the air in our shared lab stung the eyes. That mishap turned the rest of us into labeling and sealing enthusiasts overnight. The lesson stuck—sloppiness in storage costs more than lost product. It eats away at confidence and trust in the workspace. If something can irritate your lungs at trace levels, it deserves your respect, not your shortcuts.
Practical Steps for the Real World
Lock up the bottle in a chemical cabinet, and park that cabinet in a place nobody passes unless they belong there. Keep inventory up to date with regular checks. If the container degrades, replace it early. Don’t store more than you use—a hoard of hazardous compounds just expands the target should something go wrong. Clear protocols and staff who take them seriously form your best shield. In my years handling tricky reagents, habits like double-checking seals, refreshing labels, and treating every container as a potential hazard have never felt like wasted effort.
Moving Toward Safer Labs, One Step at a Time
The importance of proper storage habits is plain enough for anyone who has ever opened a chemical supply cabinet. It isn’t about paranoia, it’s about realism. These rules don’t just guard health—they shield reputations and keep good science moving forward. Handling 2',4'-Dichloroacetophenone wisely comes down to everyday choices, shaped by respect for both the material and the people alongside you.
Understanding the Chemical
2',4'-Dichloroacetophenone gets dug up in a lot of places: research labs, sometimes even manufacturing. Most people don’t recognize the name, but few realize this chemical once popped up in tear gas formulations. So, it isn’t just one of those obscure compounds in textbooks; it finds its way into situations that touch lives and surroundings.
Hazards to Human Health
Direct exposure is anything but pleasant. This substance easily irritates eyes and skin. A splash or a whiff can spark burning, tearing, coughing, or shortness of breath. I remember my university lecture when we discussed the impact of chemicals like this on the mucous membranes — the professor didn’t need to say much after showing images of corneal burns and blistered skin. Even brief exposure raised eyebrows in the class. Chronic exposure nudges its way into far riskier territory, with possible nerve and liver concerns.
The United States Environmental Protection Agency (EPA) lists dichloroacetophenones with a toxic profile. No one recommends breathing this chemical without serious protection. The Centers for Disease Control and Prevention (CDC) has guidance that any release, even a tiny amount, should be treated as a significant health event. Short-term symptoms often mask deeper, longer-lasting issues. Plus, people who have asthma or other breathing troubles are likely to experience fiercer reactions.
Accidents happen. Spills or leaks don’t stay confined in labs. Improper handling can send vapors into shared spaces, even outside schools or businesses. Proper training and solid safety gear aren’t optional here—they’re necessary to keep people safe.
Environmental Effects
It isn’t just the people holding the chemical that face risks. If it heads outside, it poses problems for water, soil, and wildlife. Once it hits the ground or waterway, organisms feel the hit. Fish and small aquatic creatures absorb toxins faster than land animals. Studies show certain compounds like this one stick around, breaking down slow and sometimes making byproducts with their own hazards.
If someone dumps this into the wrong place or an accident sets it loose, local plants might stop growing right, and people living nearby could spot changes in water taste or quality. Over time, repeated low-level contamination has a habit of piling up. Living next to sites that used or disposed of such chemicals always keeps communities on edge. Once a year, someone discovers a chemical dump or leaky drum and has to call in cleanup crews.
Reducing the Risks
Strong regulation counts. Records show that industries using strictly controlled storage and disposal procedures report far fewer incidents. Investing in safer substitutes goes a long way, too. Many groups now look for less toxic alternatives, especially in research or riot-control work. My own experience in safety committees taught me you can’t trust a label or Material Safety Data Sheet alone; on-site training saves more trouble than any set of written rules. When people actually practice spill responses and proper storage, events drop off fast.
Public awareness stays just as important as government rules. People deserve to know what chemicals move through their neighborhoods. Emergency plans—honest, practiced, and clear—build real trust and safer outcomes. It's not about sounding alarms. It's about real steps that cut risks and keep health—and the local stream—cleaner for everyone.