2,4,6-Trifluorophenol: A Deep Dive
Historical Development
Back in the mid-20th century, chemists started to realize that swapping out hydrogen atoms for fluorine on aromatic rings gave their compounds a whole new set of properties—volatility dropped, reactivity could be dialed up or down, and biologically, everything shifted. 2,4,6-Trifluorophenol stepped onto the scene through curiosity-driven research, in part by pharmaceutical and agrochemical needs. Scientists were looking for ways to tweak existing molecules to improve performance in new drugs and advanced materials. Before long, standardized methods of producing it started spreading, drawing on a mix of academic and commercial drive.
Product Overview
2,4,6-Trifluorophenol can be a game-changer depending on the application. A white or off-white solid, it often comes as fine crystals, packing a serious punch due to its altered electron distribution from the three fluorine atoms. Labs and industries keep it in tight, sealed containers, not just for safety, but to avoid moisture grabbing onto it. Scientifically, it represents a way to fine-tune molecules before sending them into real-world use—it’s a tool showing up on benches from pharma to electronics.
Physical & Chemical Properties
The molecule weighs in with a molecular formula of C6H3F3O and a molar mass around 148.08 g/mol. The three fluorine atoms shift its melting point up, typically past 40°C, and give the phenol a certain tenacity on the bench—volatile, but manageable. It’s not sociable with water, but mixes well with organic solvents like ether and chloroform. Strong electron-withdrawing effect from the fluorines means both acidity and chemical stability shift, and that difference is what often matters in synthesis or final product stability. It’s more acidic than regular phenol—now you’re tweaking reactivity just through a careful atomic swap.
Technical Specifications & Labeling
Precision wins in labeling chemicals like this, including details such as purity (usually upwards of 98%), melting point, lot number, and the date of manufacture. Certificates come with most reputable sources, noting analytical methods, impurities, and the source. This level of detail is essential for traceability: researchers and quality assurance staff count on these numbers both to match regulations and to get predictable results in studies and production runs.
Preparation Method
Synthesis starts from the nitro- or chloro-substituted trifluorobenzene; most routes use nucleophilic aromatic substitution to install hydroxyl groups on the ring once the three fluorines set, often with metal catalysts and bases to facilitate the move. Other experiments use copper-catalyzed hydroxylation steps at moderate temperatures to pin the hydroxyl group onto the ring. Each step demands careful environmental and procedural controls—the choice of solvent, reaction time, and temperature swings outcomes dramatically, both in terms of yield and purity.
Chemical Reactions & Modifications
2,4,6-Trifluorophenol acts as an intermediate in more advanced synthetic efforts. It can form ether or ester derivatives, which chemists use to assemble complex pharmaceuticals or materials science prototypes. One famous path takes advantage of the acidity to replace the hydroxyl group, giving way to various activated derivatives. Another direction: the fluorine atoms can direct other incoming groups during aromatic substitution, a trick that lets chemists build precisely modified compounds for testing in drug discovery or smart materials research.
Synonyms & Product Names
You’ll find it sold under several names: 2,4,6-Trifluorophenol, 1,3,5-Trifluorophenol, and, in some catalogs, simply “TFP” (not to be mixed up with triphenylphosphine). Keep an eye on paperwork and labels—the CAS number (somewhere along the lines of 1686-59-5) offers more certainty than names, which might shift between suppliers or in different countries.
Safety & Operational Standards
This compound doesn’t play nicely with skin or lungs—most people who’ve handled it without full PPE have regretted it. The molecule’s volatility means lab users run with gloves, goggles, and, when possible, keep work in the fume hood. If spilled, clean-up requires proper waste containers and ventilation. Material safety data sheets stress these points for good reason; chronic exposure isn’t thoroughly mapped, but routine exposure never improves job security or long-term health.
Application Area
Demand traces back to specialty pharmaceuticals, agrochemicals, and advanced electronics. Its acidic phenolic hydrogen gives medicinal chemists a handle to build enzyme inhibitors or drug candidates with specific metabolic properties. In crop science, the trifluorinated structure brings pest resistance and environmental stability to active ingredients, giving products an edge in challenging climates. Out in electronics, specialists explore its role in polymer design, where stability toward heat and chemicals matters. Each field depends on the tunable reactivity the molecule offers—flavors of the final product come from swapping these atoms precisely.
Research & Development
R&D teams lean hard on 2,4,6-Trifluorophenol for its “building block” qualities. Drug hunters substitute it into leads to block metabolism and extend half-life. Polymer chemists react it to boost dielectric properties in next-gen circuit boards. Environmental chemists tinker with degradation studies to test persistence and break-down pathways. New papers emerge every year as the field grows, sharing structural tweaks, new synthetic access routes, and bioactivity profiles so that teams can push the limits of how this building block performs.
Toxicity Research
Work on toxicity doesn’t offer an entirely reassuring picture. The molecule proves moderately toxic by ingestion or inhalation, irritating both airways and mucosa. Animal tests reveal central nervous system and liver sensitivity at high exposures. No tested chemical gets an unconditional green light, and the fluorinated ring demands particular environmental and occupational care. Regulatory agencies watch closely for persistent organofluorine compounds in soil and water, and labs must keep protocols tight to minimize unintended release or uptake by staff.
Future Prospects
Industry turns toward fluorinated compounds for good reason—stability against both heat and chemical attack, plus a toolkit for making innovative pharmaceuticals and electronics. Advanced methods in green chemistry push for cleaner, safer synthesis and easier recycling of fluorinated waste, promising to ease some environmental impact. Research teams explore novel ring modifications and substitution patterns, hoping to unlock new biological activities or material properties. As regulations catch up to emerging science, future development will need to prove both molecular ingenuity and responsible stewardship.
What Makes 2,4,6-Trifluorophenol Stand Out
2,4,6-Trifluorophenol doesn’t show up in household labels or flashy marketing campaigns, but its fingerprint touches some of the key industrial fields shaping modern technology. The unique thing about this compound is the combination of its three fluorine atoms attached to a phenol ring. This structure brings a punch of chemical stability people in the industry count on, especially in pharmaceutical and materials research. Whenever I talk with colleagues in chemical manufacturing, this molecule stands out for its reliable performance in tough environments.
Chemical Reactions and Synthesis
In organic synthesis, chemists use 2,4,6-Trifluorophenol to build complex molecules that wouldn’t come together so easily without its quirks. The phenolic part acts as a reactive partner in making esters and ethers, mostly through nucleophilic substitution. For chemists, it’s not just a raw material—it serves as a tool to create intermediates that pave the way for drugs and agrochemicals.
One good example comes from the pharmaceutical industry. Medicinal chemists choose this compound when they need to introduce fluorine atoms into candidate drugs. Adding fluorine tends to boost a molecule's stability in the body, slowing breakdown by enzymes. Betas blockers, anti-inflammatories, and some cancer drugs benefit from this stabilizing effect. My experience in the lab taught me that even a small structural tweak can make or break a medicine’s effectiveness or safety.
Material Science Applications Explored
Electronics and polymer researchers lean heavily on 2,4,6-Trifluorophenol. When designing advanced materials, fluorinated building blocks play a pivotal role. This particular compound delivers both heat resistance and chemical inertness—properties vital for high-performance coatings, sensor films, and certain engineering plastics. Gadget-makers and aerospace suppliers seek out materials that keep performing year after year, through heat, cold, or chemical spills. These materials sometimes start life in a beaker with 2,4,6-Trifluorophenol at the bottom.
Environmental and Health Factors
As with most fluorinated organics, questions get raised about exposure and persistence. 2,4,6-Trifluorophenol doesn't linger in everyday environments to the same extent as some notorious ‘forever chemicals’, but people who handle it still need solid training and proper protection. In the lab, I always made sure to keep gloves and ventilation in play. The larger concern crops up in how waste is treated—since incomplete combustion or poor disposal methods can release toxic byproducts. Regulatory bodies keep a close watch when manufacturers scale up, to avoid repeating the mistakes made with older, more persistent fluorinated chemicals.
Improving Safety and Sustainability
Some chemistry groups actively work on greener synthesis routes. Switching to more efficient catalysts, reducing solvent use, and developing recovery processes all cut down on waste and risk. Each step taken toward tighter controls and smarter waste management protects workers, the local community, and the environment. With the push toward sustainable chemistry gaining ground, I expect to see new production methods that keep both utility and safety intact.
Looking Ahead
Industries won’t turn away from 2,4,6-Trifluorophenol anytime soon. Its key properties make it too valuable for the creation of medicines and new materials. Demand for safer and cleaner pathways will keep growing, as stricter rules and new research challenge manufacturers to find better approaches. Those improvements—grounded in research and backed by real-world data—will shape how essential chemicals like this fit into a changing world.
The Makeup of 2,4,6-Trifluorophenol
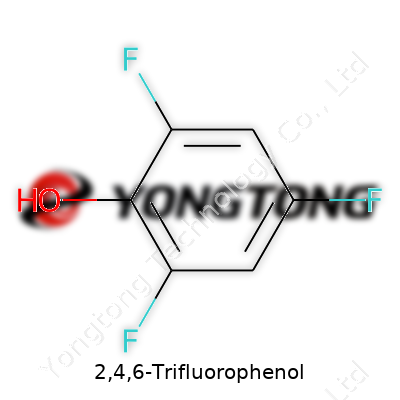
People who spend hours wrestling with aromatic chemistry tend to come across 2,4,6-Trifluorophenol. At first glance, this compound doesn’t look all that different from other substituted phenols. But there’s an edge to it: three fluorine atoms anchor themselves to the benzene ring in the 2, 4, and 6 positions, changing its properties in some serious ways. The formula goes like this: C6H3F3OH. It’s a simple equation that packs a punch.
The structure itself calls to mind the classic phenol scaffold. Picture a benzene ring—six carbons locked in that familiar hexagon—bearing a hydroxyl group (–OH) at one position and trading three hydrogen neighbors for fluorine atoms at the 2, 4, and 6 spots. Chemists love the symmetry in this molecule. The fluorine atoms aren’t just there for show; they carve out electron density from the ring, shifting the acidity of the hydroxyl group and strengthening resistance to certain reactions that would otherwise chew up plain phenol.
Why Structure Matters
In real-world settings, chemical structure goes further than fulfilling textbook curiosity. Fluorine throws a wrench into ordinary reactivity. A molecule like 2,4,6-Trifluorophenol shrugs off typical substitution reactions, showing extra stability where other phenols give out. Even on the practical side, its lower nucleophilicity means that it doesn’t react as quickly or in the expected ways—a trait that synthetic chemists keep in mind when plotting out pathways for pharmaceutical ingredients or specialty materials.
The formula alone doesn’t tell the whole story. Three fluorines stubbornly holding on to that aromatic ring change not just the reactivity but the safety profile too. Fluorinated organics sometimes linger in the environment. They carry a reputation for being tough to break down, raising eyebrows among folks focused on greener synthesis or pollution issues. Chemists, myself included, have learned to weigh these design choices with a sense of social responsibility.
Considering Real-World Uses and Hazards
I’ve seen 2,4,6-Trifluorophenol show up in labs designing agrochemicals and next-generation drugs. The fluorinated design often makes candidate molecules more resistant to metabolic breakdown. This can extend the shelf-life of a product, or keep a medication potent while working through a patient’s system. Of course, designing these molecules comes with a trade-off: disposal and long-term impact become especially important points to address.
This is why teams working with anything fluorinated have started revisiting their protocols for waste handling. Instead of sending leftovers down the sink, we’re bottling and carefully neutralizing or treating the waste. This attention to detail sits at the intersection of chemical structure and community trust. It calls for an awareness that every molecular tweak ripples outward far beyond the beaker.
Solutions to Keep Science and Safety in Balance
Better solutions start with education and transparent research. Teams need to train not just for efficiency in synthesis, but also in responsible disposal. Alternative green solvents or new synthetic strategies might ease the environmental cost of working with heavily fluorinated compounds. Regulators and researchers should keep science open, tracking new findings on the safe use and eventual breakdown of these types of molecules.
2,4,6-Trifluorophenol isn’t just another string of atoms. Its structure highlights the impact that a few small tweaks can have—for industry, the environment, and beyond. Chemists who pay attention to these details help ensure that progress travels hand in hand with responsibility and trust.
Decoding a Chemical's Risks
2,4,6-Trifluorophenol might not sound like something you’ll run into at the grocery store, but it pops up in research labs and chemical plants. Like many industrial compounds, it’s got both practical uses and a side you don’t want to ignore. This is not a chemical to take lightly, either at work or out in the environment.
What Happens with Exposure?
In my years working in analytical chemistry, even small molecules can pack a punch, and 2,4,6-Trifluorophenol fits that description. Its structure has three fluorines attached to a benzene ring, which already suggests you’ll want gloves and goggles before handling it. The real concern comes with contact. Direct skin exposure can lead to irritation. Breathe in its vapors, and you can expect headaches, dizziness, and breathing problems. The substance’s volatility means it turns to vapor fairly easily, and that’s often how it finds a way into the body apart from accidental spills.
Workplace Safety Practices
Lab safety rules exist for a reason. When handling 2,4,6-Trifluorophenol, good ventilation and solid chemical fume hoods are friends, not luxuries. Splash goggles, gloves made of nitrile, and lab coats make a real difference since this isn’t the time to go bare-handed. Anyone working with it needs solid training and a track record of following procedures. Many labs also use closed systems to minimize vapor and accidental spills. Awareness of symptoms of chemical exposure—like sudden coughing, eye irritation, or dizziness—can make the difference between a bad day and a trip to the hospital.
What About the Environment?
This isn’t just about chemistry labs. Careless disposal turns a personal hazard into an environmental one. Compounds with multiple fluorine atoms resist breakdown in soil and water, so they stick around once released. Other fluorinated compounds have a reputation for bio-accumulating up the food chain. Fish, birds, mammals—none break these molecules down quickly. Based on what I’ve seen with similar compounds, even rare spills in water can cause trouble for aquatic life. Wastewater treatments do a poor job at scrubbing these out.
Regulations and What We Can Do Better
Countries like the US and those in Europe rank chemicals by their risks and set clear disposal rules. Still, I’ve seen enforcement gaps, especially with small-scale operations or underfunded facilities. Academic environments sometimes skip formal hazardous waste training, passing up on a critical checkpoint. To improve, people use more precise tracking of chemical inventories, careful record-keeping, and invest in green chemistry substitutes where possible. For manufacturing, switch to closed-loop systems and recycle solvents when it makes sense. At the community level, people voice concerns when they spot illegal dumping, urging regulators to push back against corner cutting.
Bottom Line: Respect Over Fear
2,4,6-Trifluorophenol isn’t evil, but it demands respect. Personal protective equipment, solid waste management, and accountability at every step of handling protect both people and the places we live. We’ve learned a lot from mistakes made with now-banned persistent chemicals. This one deserves the same scrutiny, so no one looks back in a decade wishing we’d paid more attention.
Understanding the Chemical
2,4,6-Trifluorophenol carries a mouthful of a name most people haven’t heard outside a lab, but its presence in the lab isn’t rare. This compound, used in many chemical syntheses, gives off a pungent odor. Its sharp scent alone can tell you: respect is necessary here. Even folks used to handling strong-smelling chemicals stop and think twice before uncapping a bottle of this one.
Personal Protective Equipment: Suit Up the Right Way
Latex gloves always seem to pop up on hands in the movies, but nitrile or neoprene works better for 2,4,6-Trifluorophenol. This stuff seeps through some materials; go with gloves that resist strong solvents. Lab coats shouldn’t be optional—spills stain skin and clothing fast. Eye protection isn’t just for splashy experiments. Even vapors or a loose droplet sting and damage eyes. Good lab goggles wrap around the face and stop trouble from sneaking in at the edges.
Ventilation Matters
Years back, I spent a summer in a lab where air barely trickled out of rusty vents. One bad fume hood turned a regular day into a coughing fit. Proper ventilation keeps the air clean and clears out vapors before they collect. 2,4,6-Trifluorophenol gives off fumes that can irritate throats and lungs. Working in a fume hood or well-ventilated workspace makes a world of difference. Open bench tops make exposure too easy with this chemical.
Storing the Chemical Safely
Every bottle deserves a spot where it can’t tip over or break easily. Glass with tight-fitting caps works well. Plastic bottles just don’t hold up over time with certain chemicals. Light and heat push tricky compounds to break down or react, causing pressure to build or fumes to increase. A cool, dry cabinet away from heat sources, with the bottle wrapped up or inside a tray, keeps accidents in check.
Don’t mix it up with acids, alkalis, or oxidizers. Even minor reactions can create messes hard to clean up. Store similar substances together rather than lumping every bottle in one big chemical closet. Labels need to be clear and visible, not faded or peeling.
What to Do in Case of a Spill
Drops on the bench? Small spills can snowball if ignored. Absorb with sand or an inert material, then sweep up and toss the waste in a container made for hazardous chemicals—regular trash won’t do. Ventilation clears vapors, and wiping up the area with the right cleaner finishes the job. Don’t take off gloves before you’re done, and wash hands right after. Skin contact stings, and waiting too long lets problems set in.
The Human Side of Lab Safety
Training always sounds dry, but muscle memory means you act fast in a pinch. Regular practice—using spill kits, finding the eye wash station, knowing the route to a sink—makes a difference. I’ve seen nerves disappear when every step feels automatic. Talking about close calls, trading tips with coworkers, or fixing small leaks fast all build a safer routine. Nobody starts the day expecting trouble, but small habits protect everyone.
A Few Solutions for Better Safety
Label bottles before the first drop goes in. Refresh training twice a year, not just once. Rotate storage locations to avoid old stock getting forgotten and leaking. Back up physical storage with digital tracking; barcodes work faster than pen-and-paper logs. Encourage open reporting so mistakes or near misses get solved, not ignored. It takes time and effort, but it prevents bigger problems down the road. Nothing beats walking out of the lab healthy after a long day with tough chemicals.
The Purity Game: Why It Matters
Dealing with 2,4,6-Trifluorophenol in the lab brings up plenty of questions about where to draw the line with purity. Most researchers expect at least 97% or 98% purity if the goal involves organic synthesis or analytical work. A slightly higher grade, usually clocking in at 99% or above, shows up in more demanding applications, like pharmaceutical research or advanced materials. At lower purities—let’s say around the 95% mark—users notice impurities can affect how reactions run. Stray compounds or leftover solvents introduce variables nobody wants, especially considering the cost and labor of repeating failed syntheses.
Two percent may not sound like much, but when dealing with fine-tuned catalysts or bioactive molecules, a lot can happen in that gap. I’ve seen researchers skip the “lower” purity stuff unless price or project constraints force their hand. Saving some cash upfront often means paying for troubleshooting and cleanup down the road. If you need to trust your results, you stick to the purest material available, and that’s usually how the top researchers approach it.
Packaging Sizes: From Gram to Kilo
Commercial suppliers offer 2,4,6-Trifluorophenol in a range of packaging sizes. The size usually depends on whether you’re buying for a university lab, pilot plant, or larger manufacturing facility. At the smallest end, customers pick up bottles as little as one gram or five grams. This is what academic researchers and startups typically choose. These small packs keep things affordable and reduce waste if the compound doesn’t actually fit the process.
For those running a pilot project or scaling up a synthesis, larger bottles make sense. Suppliers regularly offer 25-gram, 100-gram, and even up to 500-gram packs. Factories and larger institutions might order jars that hold a kilo or more. With regulatory paperwork, chemical safety protocols, and price breaks on big orders, kilo sizes attract bigger operations confident in their process.
Packaging choices don’t stop at size. The best suppliers take care to use high-quality containers—amber glass or HDPE to keep the compound stable and dry. For sensitive materials, extra care with packing materials means fewer headaches in storage or shipping. Laboratories with limited resources always look for smaller packs; it cuts down on both hazardous waste and budget bloat. On the flip side, larger packs encourage cost savings and consistent batches for those running the same process across several runs.
Pitfalls and Solutions
Too often, a lab buys cheap, low-purity material only to chase down strange reaction outcomes or spend more time purifying the end product. Investing in high-purity trifluorophenol up front helps keep processes predictable and limits retracing missteps. Suppliers that provide traceable analytical data—think NMR, GC-MS, or HPLC results—offer more than just a number on a label; they build confidence that the chemical fits strict standards.
It helps to work with vendors who support documentation for quality, provide reliable customer service, and stay transparent on safety data. Building a relationship with suppliers pays off, especially if you need documentation for regulatory filings or scaling up a project. If storage is an issue, talk to the supplier about shelf life and packaging recommendations. It’s worth paying extra for smaller, fresher aliquots if you only use the material occasionally rather than risk degradation during long storage.
Final Thoughts
Keeping a close eye on purity and choosing the right packaging size for 2,4,6-Trifluorophenol isn’t just about ticking boxes. The right choice saves time, money, and headaches—something every scientist and process engineer can appreciate. With good sourcing and common sense, labs get consistent results while maintaining safety and compliance.