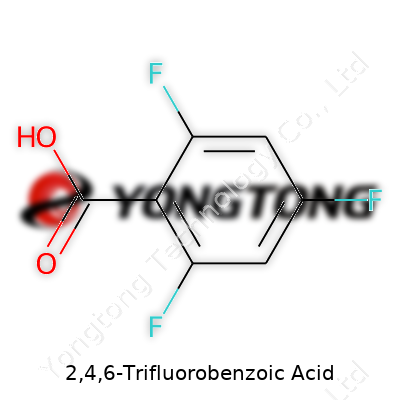
2,4,6-Trifluorobenzoic Acid: A Clear-Eyed Look at Its History and Future
Historical Development
Chemists started looking into fluoroaromatic compounds in earnest as early as the 1950s, with the aim of adding new building blocks to drug and material pipelines. 2,4,6-Trifluorobenzoic acid took shape in this landscape as a clever twist on the classic benzoic acid, setting itself apart by slotting fluorine atoms at the 2, 4, and 6 positions on the aromatic ring. Back then, traditional benzoic acids powered preservatives and fragrances, but the trifluorinated version promised to hand researchers a more tailored tool. After early syntheses refined the core structure, labs started reporting more about its reactivity and downstream modifications throughout the twentieth century. Today, catalogues list it as both a research staple and an occasional intermediate in making more complex fluorinated molecules.
Product Overview
2,4,6-Trifluorobenzoic acid shows up today as a white solid—fine powder or crystalline. Companies offer it in bottles sealed tight, often labeled by the molecular formula C7H3F3O2, with purity above 98%. Its reputation comes from the three fluorines, each swapping a hydrogen for a strongly electronegative presence. This tweak gives it different solubility, reactivity, and stability compared to plain benzoic acid. The acid handles well for most lab applications, not crazy volatile or unstable, and it remains shelf-stable in most storage setups. Chemists often turn to it for routes needing electron-withdrawing groups or to block troublesome metabolic reactions.
Physical & Chemical Properties
This compound’s melting point comes in at roughly 163–165°C, and it keeps a sharp, sweet scent—though you likely won’t want to test that fact outside a hood. It dissolves in standard polar organics like acetone, DMSO, or methanol, but not much in water. Three C–F bonds on the ring keep the molecule rigid and resistant to many reactions. The acid group works as a moderate proton donor, showing up in titration curves around pKa 2.8–3.1. Density sits a little higher than unsubstituted benzoic acid, reflecting fluorine’s heft. Altogether, it balances between moderate solubility and solid thermal stability—practical for a broad range of bench-scale chemistry.
Technical Specifications & Labeling
Labels on vials spell out key safety data: CAS number 407-28-5, formula C7H3F3O2, and storage below 25°C, away from strong bases and oxidizers. Purity usually surpasses 98% by HPLC or NMR. MSDS sheets will warn about dust inhalation and skin contact, linking to eye and airway irritation if misused. As with most lab chemicals, familiarity with pictograms helps—look for exclamation mark, not skull-and-crossbones.
Preparation Method
Chemists build 2,4,6-trifluorobenzoic acid by working through two main approaches. Direct fluorination of benzoic acid with elemental fluorine is not practical in most settings: harsh, hard to control, and prone to unpredictable substitution. Instead, electrophilic aromatic substitution or halogen dance strategies get used, starting from 2,4,6-trichlorobenzoic acid, then swapping chlorines for fluorines through halex reactions at elevated temperatures using potassium fluoride in polar aprotic solvents. This route keeps yields up and byproducts down. Purification runs through crystallization or chromatography, followed by vacuum drying. Having worked with halex chemistry, I know the smell from those reactions lingers in the lab longer than you’d like. Monitoring purity demands both careful NMR and proper handling during workups to avoid trace contamination, which can throw off sensitive downstream chemistry.
Chemical Reactions & Modifications
The trifluoro-substituted benzoic acid stands firm against many common functional group transformations. The aromatic ring resists electrophilic attack, which can frustrate some synthetic ambitions. On the upside, the acid group stays accessible for esterification, amidation, or salt formation—opening doors to making tailored pharmaceuticals, functional materials, and more. In coupling reactions, the electron-poor ring can act as a useful test bed for cross-coupling catalysts; Suzuki or Heck reactions using the acid’s derivatives show the influence of perfluoro substituents on catalytic activity. The compound’s three fluorines also shine in radiochemical syntheses, especially with 19F-NMR as a tracking tool—offering better monitoring than typical proton NMR. My experience with fluorinated substrate reactions taught me a respect for both the stubbornness and precision they demand, nudging chemists toward reaction conditions few textbooks teach in the basics.
Synonyms & Product Names
Common lab catalogues and material safety reports refer to this material under several aliases. You'll find it listed as 2,4,6-Trifluorobenzoic acid, Trifluorobenzoic acid, Benzene-2,4,6-tricarboxylic acid trifluoro derivative (by mistake at times), or simply TFBA. These names crop up across American, European, and Chinese supplier lists, which keeps researchers on their toes during literature searches or procurement planning. In regulatory filings, its CAS number 407-28-5 carries the most weight for avoiding mix-ups with other trifluorobenzoic acid isomers.
Safety & Operational Standards
Anyone working with 2,4,6-trifluorobenzoic acid learns quickly to treat it with the same caution as any fine organic acid. Dust control, gloves, and good ventilation become habits, not just checklist items. Chronic exposure—repeated skin contact or inhalation—may sensitize users, which means routines like careful weighing in ventilated balances and swift spill cleanups reduce long-term hazards. Most incidents logged don’t come from acute toxicity but rather from mishandling powders and causing minor respiratory irritation. It underscores the idea that strong safety culture grows from discipline, not just rules. Labs following standard operating procedures—complete with PPE, spill kits, and scheduled safety reviews—rarely report more than minor incidents. My own time handling compounds like this has driven home that respect for the material matters more than bravado or shortcuts.
Application Area
2,4,6-Trifluorobenzoic acid’s story stretches across pharmaceuticals, agrochemicals, and materials science. In drug discovery, it serves as a building block for designing molecules that dodge metabolic breakdown, thanks to those C–F bonds. Fluorination can raise a drug’s bioavailability or shift how enzymes see it. In materials science, its resistance to heat and chemical attack invites use in high-performing polymers and resins. Environmental chemists sometimes use it as an internal standard or tracer molecule since 19F-NMR provides a clean fingerprint among biological matrices. Universities and startup labs chase new catalytic processes or biomolecule labeling protocols that lean on the unique chemical stubbornness of the trifluorinated ring.
Research & Development
Academic chemistry and industry R&D keep testing the boundaries. There’s visible work focused on new synthetic shortcuts which avoid toxic reagents and lower environmental impact. Computational chemists model the molecule’s interactions with enzymes, hoping to launch fresh classes of drugs for viral or cancer targets. Others hunt for greener solvents or reaction conditions, knowing that traditional halex and aromatic substitution methods often raise waste disposal questions. Analytical chemists love its sharp 19F NMR signature, making it a bright marker in metabolomics and pharmacokinetics. Young researchers entering the lab ecosystem find it both a hurdle and a training ground, learning to wrestle with activation energies and selectivity challenges that build vital problem-solving skills.
Toxicity Research
The toxic profile of 2,4,6-trifluorobenzoic acid doesn’t spark the same fears as some fluorinated hydrocarbons, but it hasn’t earned a green bill of health, either. Acute oral and inhalation toxicity in mammals comes out relatively low, but long-term effects—especially environmental accumulation—haven’t received as much scrutiny as one might expect. Rodent studies show limited bioaccumulation, but the persistent nature of aromatic fluorides raises questions for both wastewater treatment and soil impact. Regulators in the EU and North America track its usage, demanding clear labeling and controlled disposal. My own experience in chemical safety research taught me that the biggest gaps usually appear fifteen years after a compound enters mainstream use, so vigilance is essential and self-assured declarations of safety ring hollow if not checked over time by independent labs.
Future Prospects
Innovation doesn’t stand still. The toolkit of benzoic acid derivatives keeps growing, and 2,4,6-trifluorobenzoic acid looks set to stick around at the edge of discovery, especially for those building next-generation drugs or task-specific polymers. Advances in fluorination chemistry—think milder, more selective reagents—point toward easier access and lower production costs. On the regulatory side, pressure is ramping up for transparent toxicology and real environmental monitoring. Automation in synthesis may let laboratories design more precise molecules, with 2,4,6-trifluorobenzoic acid serving as a foundation instead of a sidetrack. Educators and researchers need to balance the thirst for performance with an honest reckoning about what fluorinated aromatics mean for downstream waste, worker health, and rural water systems. A molecule with chemistry this rich always finds new ways to surprise, and my time in the field reminds me that curiosity, openness to criticism, and a dose of humility help keep science moving in a direction where breakthroughs serve real needs, not just synthetic milestones.
The Details Behind 2,4,6-Trifluorobenzoic Acid
Chemistry often feels like a puzzle where each piece unlocks understanding for bigger scientific questions. One small but mighty molecule that grabs attention in biochemistry and material science research is 2,4,6-Trifluorobenzoic Acid. Its molecular formula, C7H3F3O2, points to seven carbon atoms, three hydrogen atoms, three fluorine atoms, and two oxygen atoms packed into a simple aromatic ring structure.
I spent enough time in labs to know that one extra atom can make or break a project. Here, adding fluorine to a benzoic acid base isn’t just a chemical trick. Fluorine atoms sit at positions 2, 4, and 6 on the benzene ring. This positioning drastically changes the molecule’s behavior compared to regular benzoic acid. Chemical reactions shift. Biological activity can surge or fade away. Material properties take a turn. This kind of substitution has a ripple effect you can’t ignore if you care about real innovation.
Why Researchers Get Excited About Fluorinated Compounds
Fluorine atoms make molecules tougher. That’s no small detail in drug development, where stability under heat, light, and enzymes matters just as much as how a medicine works in a body. Fluorinated acids, including 2,4,6-Trifluorobenzoic Acid, often resist breakdown and bind differently to targets than their non-fluorinated cousins. Pharmaceuticals, agrochemicals, and dyes rely on these characteristics to stick around long enough to get the job done.
I’ve seen projects delayed by material degradation. Switching to a trifluorinated structure gave manufacturers that extra edge, and that all boils down to the positioning and number of fluorines—something the formula spells out clearly. In regulatory submissions, molecular formula accuracy prevents mislabeling and accidental toxic exposures. These missteps don’t just cost time; they put lives at risk.
Gaps and Challenges Worth Addressing
Fluorinated aromatics don’t get made as easily as regular benzoic acid. Synthetic routes generate hazardous waste unless handled with strict controls. Waste from halogenated compounds lingers in the environment. Communities around chemical plants feel the impact, not just lab workers. So it’s not enough to marvel at the molecular formula. Researchers and companies have to prove they can manage these materials responsibly.
Some labs are working on greener chemistry methods. Catalysts that minimize byproducts or biodegradable precursors show promise. In my experience, meaningful change picks up only when buyers demand greener options and regulators audit waste streams regularly. Collaboration between chemists and environmental specialists is overdue.
Moving Toward Safer and Smarter Science
Anyone working with 2,4,6-Trifluorobenzoic Acid needs the knack for reading beyond labels. C7H3F3O2 may not seem transformative by the numbers alone, but the impact of fluorinated acids cuts across medicine, agriculture, and manufacturing. New synthetic methods and careful stewardship will shape how this chemistry gets used in the next decade.
Learning the structure is step one. Respecting the consequences—on people, the planet, and science itself—defines responsible progress.
Modern Chemistry’s Versatile Building Block
Step into any advanced chemistry lab and you’ll probably spot a bottle labeled 2,4,6-Trifluorobenzoic Acid. This compound draws attention because of its three fluorine atoms locked into a benzene ring. That trifluorinated structure makes it a choice material for researchers chasing new molecules in pharmaceuticals, agrochemicals, and specialty materials.
Shaping Pharmaceuticals and Healthcare
Fluorine’s quirky chemistry gives molecules unique features. Drug designers often reach for 2,4,6-Trifluorobenzoic Acid when building new medicines. Pop a fluorine onto an aromatic ring and you can tweak how the final drug breaks down in the body or how tightly it binds to a target. Doctors want drugs to survive in the body just long enough to do their job—no more, no less. With 2,4,6-Trifluorobenzoic Acid, chemists can help make that happen.
Take painkillers or treatments for cancer, for example. The addition of fluorine atoms often improves potency and helps the body process the drug more predictably. No wonder more than a fifth of all new drugs include fluorine somewhere in their structure. In my own experience working alongside early-stage researchers, fluorinated benzoic acids show up every few weeks in synthesis projects, offering fresh starting points when old templates hit roadblocks.
Steering Pesticides and Crop Protection
Farmers rely on agrochemicals to keep crops healthy. But every new pesticide must work harder to outsmart pests without sticking around in the environment forever. 2,4,6-Trifluorobenzoic Acid makes an appearance here, too, as chemists use it to build the backbone of pesticides that resist breakdown from sunlight and moisture—but not so much that they persist in the food chain.
A fluorinated ring can push a pesticide to act fast against bugs and fungi, but still break down when it’s supposed to. Regulators in places like Europe ask tough questions about chemical residues, so safer breakdown profiles are a must. Startups betting on “greener” products often experiment with fluorinated benzoic acids in pilot trials, hunting for the right balance between effectiveness and environmental responsibility.
Electronics, Materials Science, and Beyond
In electronics, fluorinated compounds help craft advanced polymers needed for high-performance displays, coatings, and insulation. 2,4,6-Trifluorobenzoic Acid shows up in a list of favorite monomers and intermediates among researchers building new materials for organic LEDs or specialty plastics. Manufacturers can tune physical properties—like resistance to heat or chemicals—by swapping in fluorine atoms at precise spots on their molecular scaffolds.
Organic synthesis teachers often emphasize how reliable fluorinated benzoic acids can be in cross-coupling reactions or as anchoring groups on sensor surfaces. Reagents like this help scientists test new ideas in battery chemistry or medical diagnostics where durability and chemical resistance are at a premium.
Looking Ahead: Sustainability and Innovation
Chemistry never stands still. Every year, more groups turn their attention to the challenge of finding safer, more sustainable ways to use and dispose of fluorinated compounds. Green chemistry trends encourage cutting out unnecessary steps, recycling reaction leftovers, and designing molecules that break down cleanly. In my view, working closely with environmental researchers and policymakers will keep 2,4,6-Trifluorobenzoic Acid relevant as new applications emerge. A balance between innovation and responsibility, supported by ongoing research and transparent safety data, helps both scientists and society benefit from what this unusual molecule can do.
What Purity Tells Us
Purity shows how much of what’s in a product matches up with the label. In the lab, purity runs deeper than just numbers on a chart. High purity in pharmaceuticals, for example, isn’t about bragging rights. As someone who’s worked with quality-control teams, I’ve seen how a few percentage points can make or break an experiment, impact someone’s health, or even change a company’s bottom line. Impurities can introduce risk, from chemical reactions you didn’t plan to the spread of harmful side effects. Every gram counts, especially if someone’s trusting a drug or a supplement for their condition.
The Look and Feel of a Product
Physical appearance means more than good looks. In industry, a batch that looks off—the wrong color, an odd texture, clumping—warns you something’s not right. In my own time working with powders and tablets, I learned to trust my eyes, nose, and touch as much as any lab report. If you’re running a research project, a strange yellow hue or a gritty feel can mean leftover solvents, moisture issues, or contamination. Even things as simple as how well a powder pours matter. Caking or stickiness can cause costly jams during manufacturing. Sometimes, companies lose millions because they ignore a change in the look or feel of their material.
Real Measurements and Real Stakes
Labs use robust tools to measure purity—high-performance liquid chromatography (HPLC), mass spectrometry, melting point tests—and not just because they want fancy bells and whistles. People’s safety, product quality, and even legal compliance all tie back to getting purity right. Appearance checks, though they sound basic, are often the first sign something’s gone wrong. After all, even the most expensive testing instruments can miss what a seasoned technician catches with a simple glance.
Problems in the Field and What Can Be Done
I’ve seen companies cut corners on testing, assuming “close enough” is good enough. It’s a costly mistake. Even minor contamination has sparked international recalls—think about instances where tainted blood pressure drugs hit the news. Sometimes the blame falls on poorly labeled raw materials or on-move storage. Here’s where solutions start: regular audits, investing in better training, and not skipping sensory checks. Automatic sensors and AI now spot subtle changes in color and texture faster than before, but there’s no replacing experienced workers. It comes down to a mix of old-school inspection and modern analytics.
Why the Details Matter
Each bump, scratch, or small shimmer in a product tells a story. In my own experience, small cosmetic problems sometimes hinted at bigger issues deeper in the system. Just last year, a batch of raw chemical with faint discoloration ended up saving our team months of troubleshooting. We avoided a major product recall. It’s easy to skim past these details, but every time a team pays close attention—to purity and to appearance—they build trust. Trust, after all, shapes how others see your work, your products, and your care for people relying on them.
Understanding the Practical Side
Plenty of us working in research or manufacturing know the headache that poor chemical storage can bring. Whether you’re running a lab in a university or handling specialty chemicals at a production site, everyone wants to avoid wasted time, spoiled batches, and emergency safety calls. 2,4,6-Trifluorobenzoic acid, with its unique set of risks and quirks, deserves careful attention so that none of these headaches show up.
What the Label Tells You—And What Experience Teaches
Every bottle comes with a datasheet where you see phrases like “store in a cool, dry, well-ventilated place.” On paper, that seems straightforward. Yet reality pushes you to pay attention to room layout, humidity, and temperature swings. I’ve personally seen research teams ignore the “cool” requirement, only to end up with sticky lumps in the container after a long summer. This acid doesn’t care about lab shortcuts. Store it below 25°C, away from heat sources and windows. Skip those sun-exposed shelves—sunlight can sometimes do more damage than folks realize.
Handling Moisture and Purity
Humidity finds its way into almost every lab and stockroom, especially in older buildings. 2,4,6-Trifluorobenzoic acid is a fine powder, and has a way of clumping up or degrading if humidity sneaks in. Dry conditions matter—a dedicated desiccator or sealed tightly-capped bottle is simple insurance. I remember one occasion where condensation in a storage cabinet ruined not just this acid, but several others nearby. That spoiled batch delayed synthetic runs for a week. Desiccant packs cost next to nothing, yet they keep your acid usable for months.
Containers: Material and Safety
Some acids can react with cheap or inappropriate plastics, leaching chemicals or even breaking down containers. Glass or high-quality polyethylene has worked well for me; they don’t interact with the acid, and they’re sturdy enough for regular handling. If someone tried transferring trifluorobenzoic acid into a reused soda bottle or food jar, cross-contamination and leaks would follow quickly. Clearly labeling every container with handling notes and hazard symbols might seem tedious until you spot someone reaching for the wrong bottle—one clear label is all it takes to sidestep disaster.
Ventilation and Safety Precautions
Chemical fumes, even from solid acids, can sneak up on staff if left unmonitored. 2,4,6-Trifluorobenzoic acid emits irritant dust, so nobody should open containers outside a fume hood or ventilated workspace. Keeping emergency spill kits handy, and regularly checking storage for accidental spills or leaks, lowers cross-contamination risk and protects everyone’s health.
Practical Solutions for Long-Term Success
Train everyone who enters your lab, not just new arrivals. Even seasoned staff forget protocols during busy weeks, so posted storage reminders and periodic training keep safety habits fresh. Digital inventory systems prevent misplacement and flag expired stock before it becomes a hazard. Taking a few minutes for regular checks saves hours spent cleaning up after a mistake.
Safe storage of 2,4,6-trifluorobenzoic acid comes down to more than ticking boxes on a checklist. Staff rely on clean, safe materials to do their work. Careful planning, regular monitoring, and clear communication make all the difference in keeping accidents at bay.
Digging Into the Substance
2,4,6-Trifluorobenzoic acid stands out because of its structure—three fluorine atoms attached to a benzene ring with a carboxylic acid group. The presence of multiple fluorine atoms often sets off a mental “hazard” alarm among those who have worked in labs or industry. The fluorine can make even simple organic molecules more reactive or cause unwanted effects in the body.
Where Things Get Risky
Getting careless with chemicals often leads to trouble. For 2,4,6-Trifluorobenzoic acid, the most pressing concerns come from its potential to irritate skin, eyes, and lungs. I remember working with similar compounds in the lab years ago. One small drop on the glove and the surface started to discolor. Even with double gloves, I caught the whiff of an acrid odor. Small exposures can lead to burning, redness, or even more serious reactions for people with sensitive skin.
Swallowing is rare in an adult lab setting, but don’t discount the risk—chemicals like this can irritate the digestive tract. Inhalation creates another layer of risk. Fine dust or even vapor from heating can make its way into nasal passages and lungs. Irritation doesn’t always fade with fresh air; persistent coughing or burning sensations sometimes need real medical attention.
The Facts: Hazards Documented
Material safety data from multiple suppliers shows 2,4,6-Trifluorobenzoic acid listed under hazardous categories. It isn’t classified as the most dangerous or volatile substance out there, but the warnings exist for a reason. People handling this compound have reported skin dermatitis from splashes. Eye contact, even brief, commonly triggers tearing, pain, and swelling. Animal tests show moderate to severe irritation when the acid makes direct contact with eyes or skin. No one wants to become the person quoted in a lab safety presentation.
Accidental spills have a way of turning routine tasks into emergencies. Fluorinated aromatics like this one sometimes decompose if exposed to strong acids, bases, or heat, sending off potentially toxic gases such as hydrogen fluoride that attack the lungs and mucous membranes. One time, a colleague called for help because a heated reaction with a related compound started releasing fumes. Quick thinking—hoods, goggles, gloves, and fast venting—kept everyone safe, but it left an impression on us all.
Practical Precautions That Work
Goggles beat safety glasses every time for powdery organic acids. Lightweight gloves—especially nitrile, doubled up—seal out most of the risk. Lab coats with snug sleeves help too. I wouldn’t work with this acid outside a functioning fume hood. Even if you don’t smell anything, that doesn’t mean vapors or dust aren’t present. Contamination spreads fast on benchtops, scales, and door handles if spills go unchecked.
Washing up after handling, not eating or drinking near work areas, and using equipment that stays in the lab seem like simple steps, but skipping any of them ramps up risk. I learned early to keep emergency eyewash and showers unobstructed. Occasional drills kept me ready in case of splashes.
People storing this substance in tightly sealed containers, away from heat and acids, report fewer accidents. It can be tempting to put off disposal, but waste labeled and handled through proper channels reduces mishaps and exposure. Even used gloves and tissues can carry low levels of residue.
The Bottom Line
2,4,6-Trifluorobenzoic acid isn’t the most notorious substance in the chemical world, but it deserves real respect. From skin burns to inhalation risk, short cuts in handling cost people their health and comfort far too often. Wearing the right gear and making a habit of strict hygiene—these small steps shape real safety culture. People often say they “learned the hard way,” and that experience shapes better, safer habits for life.