2,4,5-Trifluorobenzyl Alcohol: Deep Dive Into a Critical Intermediate
Historical Development
2,4,5-Trifluorobenzyl alcohol has a place in the story of modern specialty chemistry, with roots growing alongside other trifluoromethyl derivatives. Chemists began chasing selective fluorination of aromatics over half a century ago, right in the post-war period where organofluorines found utility beyond refrigerants and Teflon pans. Methods evolved from tedious batch syntheses toward safer, more predictable processes in university and industrial labs. Interest picked up momentum as more agrochemical and pharmaceutical products began featuring trifluorinated motifs, often unlocking significant changes in bioactivity or stability. This one, 2,4,5-trifluorobenzyl alcohol, often rides along as an intermediate, supporting a host of experiments and APIs, especially since electrosynthetic and catalytic advances made materials less scarce.
Product Overview
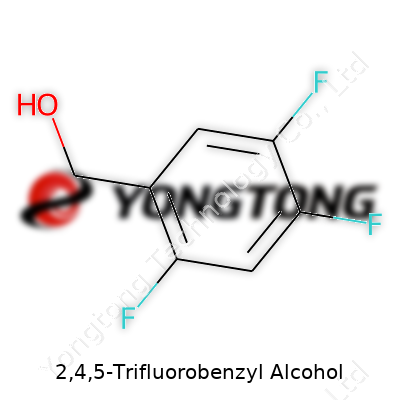
This alcohol, usually appearing as a colorless to pale yellow viscous liquid, serves roles in fine chemistry both as a finished product for research and as a scaffold for further transformation. Ask most chemists working in pharma or fine chemicals; this building block pops up on inventories from specialty suppliers. Its molecular formula, C7H5F3O, shows a benzyl backbone with fluorines on the 2, 4, and 5 positions, shaping electronic and steric parameters in a way that natural benzyl alcohols never could. Labs often order it for synthesis projects, given its useful reactivity and relative stability on the shelf.
Physical & Chemical Properties
2,4,5-Trifluorobenzyl alcohol stands out with a melting point close to room temperature and a boiling point generally above 200°C, making it manageable but not volatile under typical conditions. Water solubility runs low thanks to the trifluorinated ring, but it mixes well in most common organic solvents from ether to dichloromethane. Its density sits moderately high due to the three fluorines, and its refractive index helps analysts confirm purity with routine instrumentation. In the lab, it resists mild acids and bases but can engage in classic benzylic oxidations, reductions, and nucleophilic substitutions.
Technical Specifications & Labeling
Suppliers provide tight technical specifications: purity often exceeding 98%, with residual solvents, water content, and byproducts thoroughly controlled. Labeling requirements, especially under systems like GHS, spell out all hazards clearly—flammable liquid, harmful if swallowed, potential target organ effects, and so on—with batch-to-batch data tracked using lot numbers, manufacturing dates, and analytical reports outlining exact spectral and chromatographic signatures. That transparency forms an important part of safety culture, where even shelf labels feature hazard pictograms and careful instructions for handling and waste.
Preparation Method
Bench chemists produce 2,4,5-trifluorobenzyl alcohol mainly through reduction of its corresponding aldehyde or ester, by catalytic hydrogenation or by employing sodium borohydride or lithium aluminium hydride. Getting the trifluoro pattern right often starts upstream with selective fluorination or halogen exchange, sometimes making use of transition metal catalysts under controlled temperatures and pressures. Careful quench and aqueous workup leave behind a product that, after distillation or chromatography, fits tightly within regulatory and process quality standards.
Chemical Reactions & Modifications
Benzyl alcohols carry a reactive handle at the benzylic position, and trifluorination strongly tunes that reactivity. Chemists can oxidize 2,4,5-trifluorobenzyl alcohol to the corresponding aldehyde or acid using common oxidants like PCC or permanganate. It slides into etherification and esterification reactions without fuss, responding predictably to acyl chlorides and acid anhydrides. The presence of electron-withdrawing fluorines shields the aromatic ring from unwanted side reactions, often aiding in cross-coupling chemistry where other benzyl derivatives might fall apart. Halogenation, sulfonation, or conversion to azides and nitriles enable the creation of complex molecules for drug and crop protection leads.
Synonyms & Product Names
In catalogs and literature, 2,4,5-trifluorobenzyl alcohol sometimes goes by names like TFB Alcohol, 2,4,5-TFBA, or simply Benzyl alcohol, 2,4,5-trifluoro-. Indexes like CAS and EINECS help cut through confusion, assigning this compound a unique identity regardless of supplier, nomenclature differences, or language.
Safety & Operational Standards
People who work with this material day-in, day-out respect the potential hazards posed by volatile aromatic alcohols. Eyes, skin, and mucous membranes need full protection, as does the respiratory tract. Storage away from oxidizers, acids, and heat sources comes standard for aromatic alcohols with fluorine content. Labs and production lines install robust exhaust systems, employ spill kits, and mandate double-gloving during transfer. Regulatory guidance such as OSHA, REACH, and CLP anchor the protocols, while regular employee training keeps everyone aware of updated best practices.
Application Area
You won’t usually find 2,4,5-trifluorobenzyl alcohol on big bulk commodity runs, but it serves as a pillar in pharma and agrochemical R&D and manufacturing. Medicinal chemists harness it as a starting point for a variety of life sciences candidates where the unique combination of benzyl and trifluoro groups confers improved metabolic stability, central nervous system penetration, or target selectivity. It also supports fine dyes, specialty polymers, and materials science—especially as performance-improving additives or advanced surface treatment intermediates.
Research & Development
Across both academia and industry, this molecule draws steady attention for new synthetic methods and as a key fragment in patent filings. Recent years saw push for greener synthetic routes—maybe using water as a medium, less toxic metals, or even biotransformations with engineered enzymes. Structure–activity relationships in drug discovery projects keep this motif alive in screening decks, and computational chemists continue exploring its molecular properties in silico for everything from energetics to environmental persistence. New manufacturing improvements start in the lab but eventually streamline into scaled production, often reducing cost or improving purity, helping R&D truly pull its weight on the bottom line.
Toxicity Research
Understanding toxicity matters for every step—handling, storage, disposal, and environmental impact. Early studies indicated that most trifluorinated aromatics show lower acute toxicity than their chlorinated or brominated cousins, but vigilance remains high. At high doses, rat studies report mild hepatic effects, and in vitro assessments point towards moderate cytotoxicity depending on exposure and metabolism. Regulators keep watch for long-term effects and potential for bioaccumulation, especially as some perfluorinated compounds earned a bad reputation due to environmental persistence. Proper risk assessments stay underway both for workers and for indirect environmental exposure. Sub-chronic studies, genotoxicity screens, and fate analyses inform global standards for waste and emissions.
Future Prospects
The chemistry world keeps opening up new doors for trifluorobenzyl motifs, including promising candidates for next-generation medicines, safer pesticides, and electronics with extreme performance requirements. Rising demand in personalized medicine and smart materials means that fluorinated benzylic fragments could see wider application. Meanwhile, supply chains must adapt to secure raw materials ethically and sustainably, balancing demand with mindful stewardship. Process intensification, catalyst recycling, and greener reaction engineering all promise improved efficiency, while analytical innovations arm quality control teams with rapid, non-destructive techniques. Keeping an eye on regulatory winds will prove necessary, since greater awareness of fluorinated pollutant issues drives reform. Training, compliance, and transparent documentation will stay central as the compound’s use keeps growing and evolving with science and society.
Critical Ingredient for Specialized Chemistry
2,4,5-Trifluorobenzyl alcohol carries its weight in the world of organic synthesis. Its chemical structure brings something special to the table—a trifluorobenzyl backbone with broad reactivity for crafting more complex molecules. Walk into any lab that focuses on drug discovery, and there’s a fair chance you’ll find this compound on a shelf. Its presence signals a push toward designing molecules that last longer, resist breaking down, and pass easily through membranes—qualities prized in pharmaceuticals.
Medicine’s Need for Selective Building Blocks
Drug research needs building blocks with both flexibility and reliability. This alcohol fits that bill. Drug chemists tap into the unique properties of the trifluoromethyl group for new treatments, since swapping hydrogen atoms with fluorine often tweaks a compound’s power and how it behaves inside the body. A handful of modern antiviral and anticancer compounds trace their roots to reactions powered by this alcohol. Synthetic routes often include trifluorobenzyl alcohol as a stepping stone, letting chemists attach functional groups exactly where they want them.
Flavor and Fragrance: Tiny Changes with a Big Impact
An average person rarely realizes how small chemical edits drive the flavors and scents in daily products. 2,4,5-Trifluorobenzyl alcohol’s potency at low concentrations gives it a role in fine-tuning perfumes. Adding fluorine atoms alters odor profiles and enhances staying power on the skin. In the food industry, the same principle holds: minor molecular tweaks can separate a sharp, synthetic note from a rounded, pleasant one in a finished flavor. Not every batch of chewing gum or cologne will lean on this alcohol directly, but its influence ripples through the chain of raw materials.
Pushing Materials Science and Agrochemicals Forward
Engineers and chemists working on specialty polymers or coatings sometimes lean on fluorinated intermediates to give finished products extra resilience. Specificity matters—only certain compounds make the cut when the goal involves repellency or stability. Agrochemical researchers face a similar challenge: crops demand treatments that survive rain, sun, and high heat. In this context, 2,4,5-Trifluorobenzyl alcohol stands out as a preferred starting point for stronger, longer-lasting crop protection agents.
Weighing Challenges: Safety and Environmental Concerns
Every chemical brings risks. Handling this alcohol calls for proper ventilation, gloves, and careful storage. There’s an ongoing debate in both industry and academia about how persistent certain fluorinated compounds are in the environment. Scientists and regulators look closer at whether breakdown products build up in water or soil. Some laboratories now run routine screens for residual fluorinated materials in waste streams, trying to close the loop on pollution.
Green chemistry offers a promising direction. Researchers are developing new synthetic methods that cut down waste and improve yield. Switching to milder reagents or using catalysts to streamline reactions lowers the environmental impact of working with this alcohol. The hunt for safer disposal methods and biodegradable alternatives picks up speed every year. Demand for sustainable lab practices from both government and companies acts as a strong motivator for change.
Why It Matters
My time behind the bench taught me that progress in chemistry can hinge on compounds most people never hear about. 2,4,5-Trifluorobenzyl alcohol serves as a quiet workhorse—small, effective, and essential in turning bold ideas into products that make a difference. With careful use and continued research, it keeps shaping fields from pharmaceuticals to fragrances and beyond.
Breaking Down the Basics
2,4,5-Trifluorobenzyl Alcohol doesn’t show up in everyday conversations, but in the world of chemical research, it has a role that’s hard to ignore. This compound carries three fluorine atoms on a benzene ring, with an alcohol group attached. That molecular structure shapes how it behaves and where it can be used. Its formula, C7H5F3O, gives a direct look at what’s inside—seven carbons, five hydrogens, three fluorines, and one oxygen atom. The molecular weight sits at about 162.11 g/mol, a number calculated straight from the sum of atomic masses.
Real-World Usefulness
Tools like 2,4,5-Trifluorobenzyl Alcohol support teams working with pharmaceuticals, agricultural chemicals, and advanced materials. Fluorinated benzylic compounds bring unique properties. When I took part in lab work focused on drug discovery, these trifluorinated molecules kept popping up in screening libraries. Their fluorine atoms often improve metabolic stability and alter electronic properties in ways that the pharmaceutical industry values. The presence of the alcohol group means chemists can easily modify the compound, a bit like picking the ideal attachment point for turning a basic structure into a precise tool.
The value of fluorine goes beyond simple tinkering. Fluorine atoms on a benzene ring resist metabolic breakdown, helping potential drug candidates last longer in the body. In agriculture, these sorts of modifications often slow down degradation in soil, so the compound remains effective. My own work with crop protection chemicals showed that trifluorinated molecules sometimes lead to less frequent applications, saving time and reducing environmental exposure.
Digging Into the Technical Importance
Molecular formula and weight serve as the blueprint for safe handling, regulatory compliance, and design in new research. Chemists reference the molecular weight not only when weighing chemicals, but also when calculating how much needs to react with something else—a detail that can’t be shrugged off, since a few grams’ mistake can tank a synthesis or compromise purity. When analytical chemists run NMR or mass spec, correct identification of peaks or fragments in spectra relies on knowing these basics inside and out. I’ve seen experiments derail from a casual miscalculation here.
Knowing the exact backbone of a molecule like 2,4,5-Trifluorobenzyl Alcohol helps with more than just bookkeeping. The trifluorinated structure often signals to regulatory agencies that extra safety checks are in order. Environmental scientists flag fluorinated compounds for scrutiny because of their persistence—the “forever chemicals” problem. Careful tracking from synthesis, storage, use, and disposal can prevent problems from spiraling out of control.
Addressing Safety and Sustainability
Clear labeling with the correct molecular formula and weight supports not just compliance, but community safety. In my career, I’ve watched mishandling of chemicals cascade into delays, regulatory headaches, or actual harm. Simple details like this play a bigger role in daily safety than fancy technology or complex systems. Today, a growing focus on sustainability asks chemists to keep one eye on how such structures might stick around in air, water, or soil.
Improving education and transparency around these chemical properties matters for the next generation of researchers and anyone working with chemical inventories. Training programs, better data sharing, and honest conversations between scientists, suppliers, and safety managers can help prevent mistakes, conserve research dollars, and keep both people and the environment healthy. Fluorinated compounds like 2,4,5-Trifluorobenzyl Alcohol prove that the details aren’t just for the textbooks—they’re part of responsible science.
Understanding the Substance
2,4,5-Trifluorobenzyl Alcohol comes up in chemical synthesis labs more often than most people realize. Its trifluorinated structure calls for attention far beyond what you’d give a kitchen solvent. I’ve handled enough reactive organics to know that treating every bottle with care isn’t just red tape—it's a necessary habit.
Keeping Risks in Check
A shelf stacked carelessly says more about workplace safety than any sign on the wall. This compound’s volatility and potential for irritation mean it rarely tolerates sloppy handling. Strong odors and reactive vapors bring headaches and more if kept in poorly ventilated areas. Colleagues new to the lab laugh at procedures until they spill something or get a whiff and realize why the rules exist.
The container always matters. Polyethylene or amber glass with a tight seal works best. These options keep out light and moisture, two enemies for most chemicals, but especially for anything fluorinated. Metal lids or poorly fitting stoppers invite contamination and slow degradation if a bottle sits there for months.
Control the Climate, Control Safety
Temperature swings threaten stability, so select a spot with reliable cooling. I learned this after a warm storage room caused a runaway reaction in a different compound; since then, I lean toward cool, dedicated space for any specialty alcohol. 2,4,5-Trifluorobenzyl Alcohol responds well to refrigeration, but make sure the fridge serves chemicals only. Food and hazardous organics have no business sharing a shelf.
Forget about open shelving. A fume hood or a ventilated storage cabinet shields against unexpected splashes and minimizes inhalation risks. Even with a strong work ethic, accidents happen. I’ve seen a simple drop break a bottle, releasing vapors that had us evacuating the lab before the air cleared. A cabinet door shuts out that chaos.
Chemical Compatibility
Grouping this alcohol with oxidizers or acids invites unnecessary danger. One mentor always had her shelving laid out with bright colored tape—blue for solvents, red for oxidizers, yellow for corrosives. Easy to grab the right reagent, but more importantly, impossible to forget what should stay separated. 2,4,5-Trifluorobenzyl Alcohol stays with other stable organics, away from any box marked with the oxidizer symbol.
Labeling and Responsibility
A label makes all the difference. Generic handwriting fades or rubs off, so invest in chemical-resistant markers or printed labels. Every bottle in my lab has both the chemical name and the last inspection date. This was born out of experience: catching a bottle from a forgotten batch prevented use of a degraded sample that could’ve ruined a project.
Responsibility falls on the whole lab. Encourage regular cleanouts and inventory checks. It’s easy for new students or staff to overlook aging stock; reminders and communal checks foster habits that protect everyone. Keep emergency info—spill kits, first aid, and contacts—posted by the door, never tucked away.
Better Habits, Fewer Headaches
2,4,5-Trifluorobenzyl Alcohol demands respect, but common sense and experience shape good practice more than any checklist. Create a space that stops mistakes before they start. From the right bottle to the right shelf and the right label, storage choices reflect pride in professional work and commitment to safety—not just for one person, but for the whole team.
Everyday Exposure and Hazards
Most people won’t stumble across 2,4,5-trifluorobenzyl alcohol while grabbing groceries or walking their dog. You mostly find it in labs or in the back rooms of specialty chemical plants. Still, chemicals like this have a knack for finding a way out of their bottles. Spills happen. Ventilators quit. And a small leak often invisibly creeps up until someone’s stuck with a headache, a rash, or something a bit more serious.
Safety Data and Health Concerns
Take a closer look at safety documentation, and there’s that word—“irritant.” Breathing in fumes may cause coughing or discomfort in the nose and throat. There’s a good chance that even small splashes on the skin leave you itching or develop into a red patch. Eye contact? Expect burning and tears. Not much makes eyes water quicker than a strange alcohol like this.
Maybe more worrying, chemical studies on close relatives show some cause for concern about long-term exposure. Some benzyl alcohols lead lab animals down a rough path: liver damage, kidney struggles, or effects on the nervous system. No one wants to discover those symptoms firsthand. The trifluoro group in the molecule ramps up the potential for persistence in the environment, making cleanup tricky and turning small spills into long-lived problems.
Regulation and Workplace Practice
Government agencies keep tabs on chemicals that pose a danger. At the time of writing, 2,4,5-trifluorobenzyl alcohol doesn’t sit on the most dangerous lists alongside things like benzene or cancer-linked solvents. That doesn’t mean it’s safe for careless handling. Experienced lab workers always wear gloves, eye protection, and lab coats. They work under decent ventilation. They never eat or drink near their chemicals. Respect for these tools becomes second nature.
Many companies invest heavily in air filtration and spill response training. It pays off every time there’s an accident. Employees also need to know that some symptoms may develop slowly. Self-care starts with knowing what you’re working with and reporting problems early. A day with no accidents only counts if you’re honest about close calls and near misses.
Reducing Risks
Good practice means using strong gloves and goggles every time you handle chemicals. Spill kits must stay nearby, ready for accidents—even if they seem unlikely. Online safety sheets provide clear pointers: avoid inhalation, prevent skin contact, never mix this with strong oxidizers, and store it away from heat sources and sunlight.
I’ve seen too many new workers underestimate a colorless liquid just because it looks harmless in a beaker. It’s easy to think “nothing happened to me last week.” Still, health trouble from chemicals like this can take time to show up. People who breathe a bit of vapor day after day might not notice symptoms until weeks later. That’s why you err on the side of caution.
Looking Forward
Better chemical substitutes do exist for some roles once filled by substances like 2,4,5-trifluorobenzyl alcohol. Greener chemistry means less risk for workers, the public, and the environment. Engineers continue to search for less hazardous alternatives, and managers listen when new options cost less to store, handle, and dispose.
Chemicals rarely come with simple, black-and-white answers. Safety grows from honest training, strict routines, and a willingness to change old habits. The story of 2,4,5-trifluorobenzyl alcohol reminds us that simple caution and clear information protect lives—and that nothing beats a clean pair of gloves and good ventilation.
The Importance of Purity in Chemical Use
Working in a chemistry lab quickly teaches respect for chemical purity. Impurities tend to complicate results, create safety risks, and even throw off whole syntheses. Everyone I've shared a bench with keeps a close eye on the purity reports for specialty reagents, especially ones like 2,4,5-Trifluorobenzyl Alcohol (TFBA). TFBA shows up in all sorts of research: pharmaceutical intermediates, fine chemical synthesis, and sometimes in agrochemical development. Each field sets its bar for purity, but a number keeps coming up: 97% or greater. That’s the figure I see most often stamped clearly on the supplier spec sheets. For the more intense routes, 98% jumps in, and there’s a handful of cases where someone chases down 99% for that last bit of confidence. In many applications, trying to avoid mystery peaks on the chromatogram keeps everyone focused on what’s in that small bottle.
Packaging Sizes and Why They Matter
Ordering chemicals isn’t like grocery shopping. Too little, and work gets delayed for weeks. Too much, and unused stock expires, leaks, or ends up as hazardous waste. Providers know this, so they offer a handful of packaging options. I’ve seen TFBA typically available in small glass bottles — usually in 1-gram, 5-gram, and 25-gram sizes for bench-scale work or method development. Larger projects turn to 100-gram or 500-gram glass bottles. Some catalogs show options in amber glass to protect light-sensitive materials, which matters for stability, especially in organic alcohols. Moving up to the kilo scale, metal drums and HDPE containers come into play, but I rarely see this unless an industrial process is in full swing.
Not everyone stocks every size; sometimes, only 5-gram and 25-gram bottles ship readily from a regional warehouse. Bigger batches often call for a lead time while the supplier re-packages the material. I learned this the hard way, waiting extra days while the supplier filled a 100-gram order as a custom request.
Documentation, Safety, and Supply Chain Transparency
Google’s E-A-T principles stress the need for expertise and trustworthiness, and these matter for TFBA procurement. A reliable supplier doesn’t just sell a reagent; they should back it with a certificate of analysis listing purity (by GC or NMR), and ideally mention any residual solvents or detected contaminants. Sometimes the supporting documents cover exact methods and detection limits.
Personal experience reminds me that quick access to MSDS (Material Safety Data Sheets) is essential. Most reputable companies make these easy to download from their sites, and staff make sure every container carries a proper hazard label. Don't gamble with off-brand chemicals missing this documentation—expect headaches and safety audits.
Improving Traceability and Reducing Waste
Given the cost and environmental profiles, buyers and lab managers push for responsible purchasing. Picking the right packaging size controls inventory costs and lessens expired waste. Some suppliers started offering “green” packaging: recyclable containers, more robust seals to avoid leakage, and batch numbers for traceability back to the production run. That last item saves time in audits and meets stricter research funding requirements that focus on data reliability.
For research teams needing consistent, verified TFBA, sticking with suppliers that maintain ISO-certified processes brings extra assurance. I’ve noticed more labs refuse shipments without clear COA and batch traceability, and this trend shows no sign of fading as quality standards climb.
Looking to the Future
Every step in the supply of 2,4,5-Trifluorobenzyl Alcohol brings its own demands, from purity above 97% for clean downstream chemistry, to selecting a bottle size that fits the workflow. Standardizing specs and keeping meticulous records sits high on every smart lab’s checklist. By keeping supply chains transparent and embracing new ideas in packaging, chemists can work more safely and produce more reliable results.