2,3-Difluorophenyl Butyl Ether: Commentary on Science, Use, and Future
Historical Development
If you look back at the story of ether compounds, scientists started working with simple alkyl aryl ethers as early as the late 1800s. The ideas behind fluorination began picking up steam in the 20th century, when pharmaceuticals and agrochemicals needed greater metabolic stability, environmental resistance, and unique physicochemical tweaks. 2,3-Difluorophenyl Butyl Ether, with its niche set of two fluorines on the aromatic ring, didn’t become accessible until researchers got the hang of regioselective fluorination techniques. These advances invited fresh conversations about applications ranging from specialty solvents to the backbone for drug candidates. The work has built on organic synthesis breakthroughs that let chemists tack on functional groups exactly where they want them. Some of the drive behind developing this molecule came from the persistent quest for molecules with tuned lipophilicity, electric polarization, and metabolic profiles—for both industry and academia.
Product Overview
2,3-Difluorophenyl Butyl Ether stands out as a colorless to pale liquid, offering up a sweet spot of chemical stability and unique reactivity. Its two fluorine atoms make it particularly interesting, since selective fluorination reshapes not just a compound’s reactivity, but also how easily it passes through biological membranes or resists metabolic breakdown. This ether brings value as both an intermediate in organic synthesis and a subject for structure–activity studies in medicinal chemistry. It’s shown up in research exploring drug-like molecules, as well as specialist polymers and liquid crystals. People tend to use it not for its basic physical properties, but because of that two-pronged fluorine group, which can change how the molecule interacts in everything from biological assays to specialty materials.
Physical & Chemical Properties
On a lab bench, you’ll notice 2,3-difluorophenyl butyl ether remains clear and practically odorless, dissolving in organic solvents but resisting water. Its melting and boiling points run higher than non-fluorinated counterparts, since those C–F bonds set the bar for thermal strength. The compound’s moderate density gives it a solid feel without being unwieldy in flasks. The electron-withdrawing effect from the fluorines substantially drops the electron density on the aromatic ring, which shapes its behavior under electrophilic and nucleophilic attack. That tweak doesn’t just change the lab protocols—it also sets this compound up for novel reactivity patterns that other ethers can’t quite match.
Technical Specifications & Labeling
Researchers and chemical suppliers pin their confidence on a narrow purity range, often 98% or higher. This speaks to the kinds of applications—whether it’s scale-up for active pharmaceutical ingredient (API) intermediates or reference standards in analytical labs—where impurities can spoil outcomes. Labels won’t just list CAS numbers and formula weights, but include critical info about storage temperature, recommended handling, and expiration. Regulatory bodies look for labeling conforming to the Globally Harmonized System, which brings everyone to the same table in recognizing hazards (think: flammable, irritant, environmental warning).
Preparation Method
Behind every bottle of this ether sits a stack of synthetic chemistry. Typical routes start from difluorinated phenols, usually 2,3-difluorophenol, then run alkylation through a Williamson ether synthesis using butyl halide in the presence of a base like sodium hydride or potassium carbonate. These reactions run best in aprotic solvents, often under nitrogen or argon to stave off unwanted side reactions. High-throughput flow chemistry brings reproducibility and speed, making production less prone to batchwise error than old-school setups. The clean-up often needs careful extraction, sometimes column purification, depending on the demands for downstream purity.
Chemical Reactions & Modifications
The real charm of 2,3-difluorophenyl butyl ether comes from the new universe of reactivity the two fluorines offer. They both block and guide further functionalization. For example, the electron-deficient ring opens up unusual pathways for selective deprotonation or metallation, so synthetic chemists can build up more complex molecules in structured, predictable steps. The ether function serves as a handle for transetherification, oxidation, or even cross-coupling provided you put in the right catalytic system. Sometimes the molecule serves as a scaffold for Suzuki or Buchwald–Hartwig reactions—kicking open doors for the next generation of tailored molecular designs.
Synonyms & Product Names
On datasheets, you might spot it listed as Butyl 2,3-difluorophenyl ether, or even 1-butoxy-2,3-difluorobenzene depending on supplier conventions. Chemical catalogs will offer alternative registry numbers, but they’ll usually cross-reference the more systematic names so labs, regulators, and procurement all speak the same language. Sometimes, creative product codes hint at batch or regional sourcing—but the chemical heart stays the same.
Safety & Operational Standards
Good lab practice calls for handling this ether with eye protection and gloves, in a fume hood. It packs the typical risks you get with organic solvents: moderate irritancy, possible environmental toxicity, and flammability under open flame or spark. Storage calls for tightly closed containers, cool temperatures, and good airflow to prevent vapor build-up. Disposal of waste involves either incineration in permitted facilities or transfer to authorized disposal contractors, matching national and local hazardous waste rules. Training technicians and workers on proper response to spills—absorbent, non-combustible materials, and ventilation—keeps everyone safer. Compliance departments keep material safety data sheets available and updated, so any incident gets a timely, effective response.
Application Area
My time in academic and industrial labs showed me 2,3-difluorophenyl butyl ether often gets tapped for far more than its name suggests. Medicinal chemists use it to explore structure–activity relationships for new fluorinated drugs, since it helps tweak how a molecule gets absorbed or metabolized. In agrochemicals, minor changes on the ring can spell big differences in pest resistance or environmental persistence. Analysts find it useful as a standard in chromatography and spectrometry, particularly where selectivity for fluorinated aromatics matters. Materials researchers build from it into new polymers, liquid crystals, and fluorous phases—all leveraging the properties bumped by those two aromatic fluorines. Each discipline values its unique blend of chemical inertness, predictable metabolism, and physical traits that set it apart from plain ethers.
Research & Development
Work in R&D keeps pushing the boundaries for this molecule. Labs look at optimizing synthetic processes to cut down waste and hazardous by-products, whether that means greener solvents, flow synthesis, or catalytic methods that run at milder conditions. Scientists probe modifications on the butyl chain or phenyl ring to beef up or tune biological activity, finding new leads for antiviral, anticancer, or neuroactive agents. Research collaborations across pharma and agrochem lead to patents staking out new territory on derivatives. On the material side, groups assess how this ether stacks up in new specialty fluids or battery electrolytes thanks to its unique dielectric profile. R&D conversations draw in industry partners, regulators, and academic theorists, each with a different stake in what this one molecule could unlock.
Toxicity Research
No discussion about a fluorinated aromatic ether would be honest without straight talk on toxicity. Preclinical testing in mice and cellular models probes for both acute effects—think irritation, neurological impact, organ stress—and longer-term metabolites. Most ethers pose inhalation and skin exposure risks, and the C–F bonds mean slow degradation by natural processes, so environmental scientists look closely at persistence and bioaccumulation. Industry responds by setting stricter exposure limits in workplaces and promoting personal protection and engineering controls. Regulators hope for more data on human health impacts, since metabolic and excretion profiles differ across species. These checks are not just boxes to tick—they shape whether and how this ether hits the market in new applications.
Future Prospects
Anyone tracking emerging trends would notice the growing hunger for new fluorinated molecules in pharmaceuticals, advanced materials, and sensing applications. 2,3-Difluorophenyl butyl ether sits at the crossroads of these markets—offering starting points for synthesis, tweaking bioactivity, or enabling fresh properties in polymer development. Advances in sustainable synthesis promise to make its preparation cheaper, faster, and greener. Researchers still look to unlock subtle modifications on the ether or aromatic ring, aiming for next-level functionality without driving up toxicity or environmental cost. The push toward better analytical techniques and more targeted drug or pesticide development rests on scaffolds like this one. If society keeps needing more efficient molecules and smarter materials, the value of such tailored ethers will only grow, with scientists leading the way through practical, evidence-based innovation.
Understanding the Structure
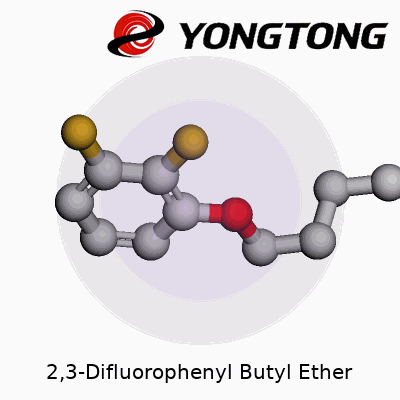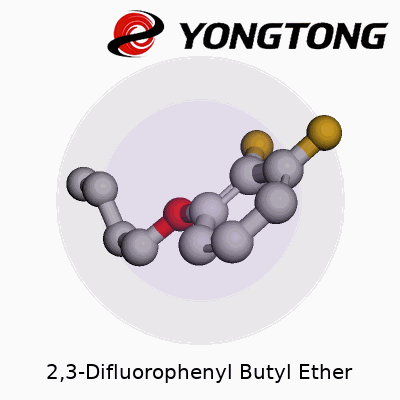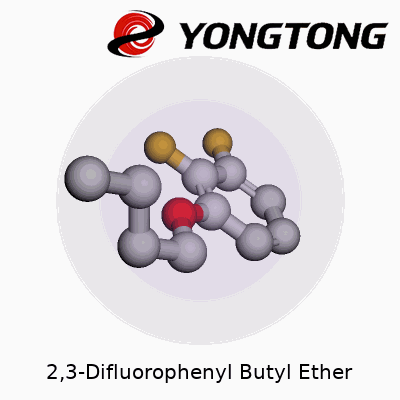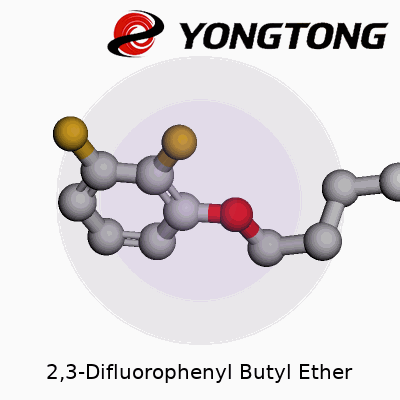
Every time I run into a compound like 2,3-Difluorophenyl Butyl Ether, my mind starts sketching out the molecular skeleton. Start at the base: a phenyl ring holds six carbons, tight in a benzene arrangement. The “2,3-difluoro” tag means two fluorine atoms replace hydrogens at the 2 and 3 positions on that ring. Attach an oxygen somewhere, according to ether rules, and link a butyl group (four straight carbons) right at that oxygen. These structural bits decide what the molecule does, how it reacts, and where it ends up in any chemical setting.
Counting the Atoms
Count the puzzle pieces: the benzene ring (C6H5), the two fluorines swap for hydrogens, an extra oxygen, then a straight butyl chain (C4H9) gets tacked on. Piece those together and the numbers stack up. Six from the ring, four from the chain—ten carbons in total. Hydrogen count drops thanks to the two fluorine swaps and the loss of another for the ether bond, setting it at twelve. Oxygen gets its spot as the single bridge between chain and ring. Fluorines land as two, per the difluoro part. So, the formula lines up as C10H12F2O.
Significance for Chemists and Industry
Pinning down a molecule’s formula goes beyond trivia for chemists. I recall handling similar ethers in the lab, where not knowing exactly what’s present can mess up reactions, safety protocols, or waste disposal. Information about every atom affects toxicity, flammability, and interaction with living systems. For anyone running a research project or scaling up new compounds for pharmaceuticals, accuracy means everything. Getting the count wrong skews dosing in drug synthesis, confuses spectra interpretation, throws off safety data sheets. Even routine quality control relies on knowing what should—and shouldn’t—be in a test batch. Regulatory checks in the US and internationally demand exact records.
Challenges for Learners
I’ve seen students and even experienced folks trip up, overlooking a hydrogen or not spotting how fluorines alter the composition. Small shifts in structure turn into big changes in behavior. Two fluorines instead of one—now you’re dealing with a very different set of electronic properties and reactivity. Ethers look harmless, but switch up the atoms and you find new boiling points, solvent powers, rates of breakdown in the environment.
Promoting Best Practices
Training matters. Getting structure–formula relationships right depends on constant practice and solid tools. Drawing out structures by hand works, but today, using digital molecule builders and checking against reliable databases like PubChem or ChemSpider prevents countless mistakes. Many of us started out scribbling on napkins and running to senior scientists for double-checks. Now, most research groups set up software checks and cross-reference every new compound entry for their logs. Anyone in teaching or mentorship roles needs to drill that habit—don’t assume, don’t eyeball it, always verify with published literature or trusted resources.
Moving Precision Forward
Watching the field evolve, I see more open sharing of chemical data and compound registries. That openness lowers barriers and errors for the next generation of scientists, tinkerers, and inventors. The formula for 2,3-Difluorophenyl Butyl Ether—C10H12F2O—shows up as a simple count on paper, but getting it right anchors every safe and useful application in the real world. Care with molecules pays off far beyond the lab bench.
What Makes 2,3-Difluorophenyl Butyl Ether Useful
2,3-Difluorophenyl Butyl Ether doesn’t show up on every company’s reagent shelf, but those who know it see its value. I learned about its unique grip on chemical reactivity during a stint working with medicinal chemists searching for the next promising molecule in drug discovery.
The 2,3-difluorophenyl backbone brings more than just molecular bulk. With two fluorine atoms nestled onto the aromatic ring, the ether functions as an essential scaffold in pharmaceutical research. Researchers often look for substitution patterns that help tweak the bioactivity, improve absorption, or boost metabolic stability. This fluorinated ether caught my attention because it achieves all three.
Pharmaceutical Building Block
Drug companies often prioritize fluorinated aromatics, and for good reason. These groups can strengthen a molecule’s resistance to metabolic breakdown in the liver, extending its activity inside the body. The butyl ether portion broadens solubility and makes the compound more accessible for downstream synthesis. In practice, teams in early-stage drug R&D have incorporated 2,3-difluorophenyl butyl ethers as starting materials and intermediates. Generating analogs with small changes to the alkyl chain or alternating the fluorine positions can reveal novel properties.
One prominent story: a friend’s project on kinase inhibitors began with difluorophenyl ethers. By adding fluorines and adjusting the side chain, they improved binding to the target enzyme and reduced unwanted interactions elsewhere. Articles in journals like Journal of Medicinal Chemistry have repeatedly highlighted similar strategies.
Crop Protection Chemicals
Agrochemical development borrows tricks from pharma. Here, 2,3-difluorophenyl butyl ether can anchor selective herbicides and fungicides. The bulkiness and stability conferred by the difluoro coupling often mean less frequent application or lower overall use. Field trials I read about in Pest Management Science showed difluorinated ethers holding up in tough soil and weather. Farmers gain crop protection that persists longer, and sometimes shows less impact on non-target plants or animals.
Organic Synthesis Intermediate
Few people outside the specialty chemical industry talk about boron coupling reactions and etherification. Yet, those reactions run the engines behind new materials and flavor molecules. In one pilot plant lab, I saw chemists using 2,3-difluorophenyl butyl ether as a precursor in building block pipelines. The molecule’s structure unlocks new reaction routes, making it a practical choice for forming complex, highly fluorinated compounds. Whenever someone in the team needed to add a difluorophenyl group without sacrificing reactivity elsewhere, this ether sat near the top of their order list.
Potential Challenges and Solutions
Nothing in industrial chemistry works without trade-offs. Large-scale synthesis of difluorinated aromatics often creates toxic byproducts. Environmental impacts draw criticism—and rightly so. New green chemistry approaches now try to use safer solvents and milder fluorinating agents, which can cut down waste and improve worker safety. Some companies encourage process engineers to recycle reagents and reduce fluorine leaks.
In the future, more research on bio-based fluorinating agents or enzyme-driven transformations could help. From my point of view, industry and academia collaborating on cleaner, lower-cost synthesis might pave the way for wider use—without the environmental cost.
Quality in the Lab: Beyond Labels
Walking into a research lab, details matter. Scientists work with chemicals like 2,3-Difluorophenyl Butyl Ether every day, and purity specifications play more than a small supporting role. Even a slight impurity can cause unexpected results, wasted time, or worse — safety risks.
Standard purity specifications for 2,3-Difluorophenyl Butyl Ether hover around 98% or higher. Leading chemical suppliers often push this to 99% for sensitive research fields. The percentage isn’t just a number on a datasheet. Behind that figure, there’s chromatography, spectroscopy, and a batch of skilled analysts running every lot through its paces. Laboratories trust those results because reliability keeps experiments on track, not just compliance checks.
Why Purity Matters in Research and Manufacturing
Contaminants disrupt reactions. Even trace impurities can add noise to analytic data, impact reaction yields, or trigger unwanted side reactions. In pharmaceutical research, anything below set purity specs means regulators and internal QA teams sound the alarm. I’ve seen teams waste weeks pinning down the source of quirks in their data, only to find an out-of-spec chemical at the root. That frustration lingers long after the batch gets tossed.
Electronics and materials science teams can face similar headaches. Take the need for ultra-clean conditions when creating semiconductors. A hint of an unknown side product in a key building block, like 2,3-Difluorophenyl Butyl Ether, can hamper device performance or shorten shelf life. Both cost real money.
Specification Details: What Labs and Buyers Check
Buyers don’t just glance at the 98% figure. They look for full certificates of analysis. These include identities confirmed by NMR and HPLC, with GC-MS to chase down those barely-there impurities. Water content, residual solvents, and trace metals often receive their own lines on the document.
If the supplier notes a 2,3-Difluorophenyl Butyl Ether batch as “98% (GC),” there’s a solid expectation that the by-products don’t mimic the target compound’s properties. Researchers may push suppliers for more info, especially where downstream drug or electronics work is involved.
The Pursuit of Clean Chemistry: Gaps and Solutions
One problem I’ve seen is suppliers using vague or incomplete specifications. Sometimes, two products both read “98%” on the datasheet, but only one holds up to closer inspection. Open communication with suppliers helps dig into the fine print behind their numbers. A good supplier will show detailed chromatograms, outline their purification process, and walk you through the impurity profile.
Labs can take matters into their own hands. Setting up in-house verification — usually HPLC or GC analysis — adds extra confidence before a key experiment. Some teams keep a shortlist of trusted suppliers known for tight specs and batch-to-batch consistency. Creating an internal database of tested lots helps steer future purchases in the right direction.
Moving Forward: Better Practices for All
Raised standards from regulators and customers continuously nudge suppliers toward better transparency. More lot-specific data, clearer labeling of residuals, and regular site inspections give buyers firmer ground to stand on. Teams can stay proactive: ask for full documentation, double-check specs, and run in-house tests. Maintaining a keen eye for detail pays off, saving time and resources where it counts. That’s the kind of vigilance I’ve seen make real differences in both research and production settings.
Understanding the Risks
Storing chemicals like 2,3-Difluorophenyl Butyl Ether means thinking about both safety and long-term stability. This compound carries a risk of fire, so flammable liquid protocols apply. I remember visiting a research facility a few years back—a misplaced bottle of a similar compound led to an entire lab evacuation when its vapors found a spark source. It’s not worth rolling the dice on safety with solvents or ethers.
Physical Storage Matters
Most technicians keep chemicals like this in a flame-resistant cabinet. Metal safety cabinets, grounded to avoid static electricity, provide peace of mind. There’s a practical side here: storing chemicals at eye level reduces the risk of accidental spills when reaching for a bottle. A colleague once kept bottles tucked away in low cabinets, but catching a sleeve on one led to a frustrating clean-up and a stern conversation about containment.
Temperature control is a big deal. Exposure to heat accelerates degradation and raises vapor pressure—if you've ever walked into a warm storage room and caught a whiff of solvent, you know something’s not right. 2,3-Difluorophenyl Butyl Ether belongs in a spot around room temperature, away from direct sunlight, and definitely out of any high-humidity areas. Excessive moisture wrestles with chemical safety. It’s tempting to cut corners, but small habits stack up. A cool, dry, well-ventilated location keeps both chemical and staff away from unnecessary risk.
Container Choices and Labeling
Chemicals love clear labeling—nothing fancy, just legible and unmistakable. Permanent ink comes in handy after a few months, so don’t trust fading markers. Only use containers compatible with ethers, like glass or HDPE (high-density polyethylene). Reactive plastic can fail over time, which can leave you with a sticky mess on your shelf and a headache for your safety inspector. The right lid goes the distance—tight-fitting and no cracked seals.
Avoiding Contamination
Lab workers face enough hazards without cross-contamination. Pour directly from the bottle without swapping pipettes between containers. Storing the compound with reactive acids, oxidizers, or bases leads to drama nobody wants. Years ago, I saw someone tuck a bottle beside a sulfuric acid container. Both looked dry and quiet on the shelf until a spill happened—and an emergency shower came next. Segregating storage by hazard class counts as more than bureaucratic red tape.
The Human Side of Chemical Storage
Too many accidents start with the phrase, “I thought it would be fine.” Storing 2,3-Difluorophenyl Butyl Ether correctly means respecting both the chemical and the people working nearby. Safety data sheets exist for a reason, so review them often. Throw out old or compromised containers before they become a bigger issue. Make safety checks a habit, not an afterthought. Hard-earned experience in the lab has taught me: shortcuts stop being convenient when things go wrong.
Long-Term Thinking
A smart storage plan prevents emergencies, protects investments, and keeps workflow steady. You’re not just protecting a bottle; you’re looking out for colleagues, property, and the environment. In the world of chemical storage, real care comes less from fear and more from consistent, thoughtful routines.
Why This Matters More Than People Realize
Working in a chemistry lab long enough, you get used to seeing those thick SDS binders jammed onto shelves, paper edges worn from anxious hands. The SDS, or Safety Data Sheet, isn’t just some regulatory hoop to jump through. It shows the risks that might come with a chemical—whether it burns, whether the fumes sting your lungs, whether a skin splash means you’d better get to the eyewash station fast. This sort of sheet saves lives. It helps folks understand what they’re working with, and how to keep the mistakes from turning nasty.
So, if you ask around for an SDS for something like 2,3-Difluorophenyl Butyl Ether, you expect to find one. Problem is, you might hit a wall. For some older or obscure chemicals, SDS sheets turn out to be little more than a technical whisper. And that’s where the trouble starts.
The Hunt for the Missing Sheet
I remember one frantic afternoon digging through cabinets and scouring supplier websites, trying to find an SDS for a compound nobody had used in years. My gloves left powder prints everywhere. In the end, all I found was a short product summary with no talk of hazards, storage, or cleanup. What struck me wasn’t just the hassle. It was the realization that newer researchers could get caught off-guard. Maybe they open a bottle thinking it’s “just another ether”—not knowing that similar compounds have flashpoints so low, a short spark could send fumes across the bench in flames.
2,3-Difluorophenyl Butyl Ether doesn’t even have much casual information floating around. Its structure has two fluorine atoms on a phenyl ring linked to a butyl group. That arrangement gives it a handful of quirks—likely some volatility, questionable stability. Fluorinated rings often mean bioaccumulation risks, and the ethers can go for the nervous system if inhaled. But without a real SDS, it’s almost a guessing game.
Why Every Chemical Deserves Its Sheet
This isn’t just about ticking a box for compliance. I’ve seen a few older researchers develop weird chronic coughs, only to trace it back to a solvent with missing safety info. Plenty of times, someone dumped a leftover sample down the sink, thinking it was harmless. Not just risky for people—potentially bad for the local water supply, too. Some fluorinated aromatics don’t break down nicely, and the waste stacks up slowly over the years.
What Can Be Done?
Regulation alone won’t solve this. Labs, suppliers, and the chemical industry need to share information more openly. Some of the best safety advice doesn’t come from the manufacturer directly, but from people who’ve actually worked with the compound. Online safety communities could help plug these holes. If you can't find an SDS, at least trade notes on hazards, safe fixes, and disposal tricks. At the same time, cataloging requirements need teeth so forgotten chemicals aren’t overlooked just because they don’t draw huge sales.
In my experience, erring on the side of caution pays off. Until someone coughs up a real SDS, stick to gloves, goggles, plenty of ventilation, and never work alone with it. Keep asking the suppliers, hound the sales reps, and maybe, just maybe, the community will catch up. Chemistry isn’t just reactions on paper. It’s built on trust—between everyone who touches the bottle.