2,3-Difluorophenol: An In-Depth Commentary
Historical Development
Chemistry thrives on understanding molecules in detail, and 2,3-difluorophenol stands out as a symbol of the rising influence of fluorine substituents in modern synthetic chemistry. Researchers started paying attention to difluorinated phenols in the late 20th century, driven by the unpredictability and value fluorine atoms bring to aromatic compounds. Early synthetic routes leaned on electrophilic fluorination—often quite challenging due to overfluorination or low selectivity. Stepwise generation, starting from difluorinated benzene cores and selective hydroxylation, marked a key turning point. As the pharmaceutical and agrochemical sectors started demanding more niche building blocks, scaling up 2,3-difluorophenol required both safer handling and greener processes. Advances in catalysis, and a better understanding of regioselectivity, slowly started making this compound a practical reality beyond small lab batches.
Product Overview
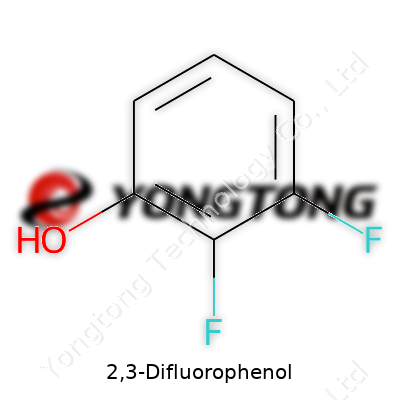
2,3-Difluorophenol is an aromatic compound with two fluorine atoms on adjacent carbons and a hydroxyl group at the ortho position. Its molecular formula, C6H4F2O, signals a relatively simple structure, but the presence of two electron-withdrawing fluorine atoms significantly alters its behavior—both chemically and in product development. Scientists and formulators turn to this molecule for its ability to introduce novel properties, including increased metabolic stability or altered electronic characteristics, in target molecules for pharmaceuticals, agrochemicals, and materials science.
Physical & Chemical Properties
On the surface, 2,3-difluorophenol appears as a colorless, sometimes pale yellow liquid at room temperature. With a boiling point near 180 °C and melting point slightly below room temperature, its volatility falls between that of regular phenol and its higher fluorinated cousins. The electronegativity of both fluorine atoms impacts acidity; the compound’s pKa usually dips below its mono-fluorinated analogs, placing it in a sweet spot for selective reactions or biological interactions that hinge on hydrogen bonding. The smell is sharp, reminiscent of phenol, but tinged with a dentist’s-office reminder thanks to the fluorine. Solubility leans on the organic side—miscible with most nonpolar and weakly polar solvents, sparingly soluble in water.
Technical Specifications & Labeling
Quality and consistency matter in any laboratory or production plant. Finished 2,3-difluorophenol, whether sourced or custom-made, typically ships at purity levels above 98% as confirmed by GC or HPLC. Free phenol content, residual solvents, and moisture require close monitoring, given the potential impact on downstream chemistry. Containers use dark glass or high-density polyethylene to minimize decomposition and contact with air or light, and labels always list the precise IUPAC name, batch number, purity, date of manufacture, and safety hazard warnings—following both GHS standards and local regulatory requirements. These include the familiar pictograms for irritant and environmental hazard, which remind handlers to treat this liquid with respect, gloves, eyewear, and adequate ventilation.
Preparation Method
Manufacturers usually rely on selective fluorination of phenol derivatives or hydroxylation of difluorobenzenes. Direct fluorination won’t work—too dangerous and likely to overreact. The main industrial routes begin with ortho-difluorobenzene, reacting under catalyzed hydroxylation or using a diazotization path from amino-difluorobenzene intermediates. Careful control of temperatures and reagents limits byproduct formation, especially unwanted polyfluorinated phenols. Flow chemistry techniques and improved catalyst recycling have pushed yields and safety forward in recent years. The old bench-top batch style is rapidly being replaced by these newer flow and semi-batch routes, which offer tighter control over heat and mass transfer, resulting in a safer and more scalable process.
Chemical Reactions & Modifications
The real value of 2,3-difluorophenol lies in its reactivity profile. Both fluorines create a benzene ring that resists nucleophilic attack at most positions, but electron density shifts make ortho and para positions to the OH group especially interesting for cross-coupling. Suzuki–Miyaura reactions or Buchwald–Hartwig aminations thrive with the right catalyst, allowing the rapid buildout of complex aromatic scaffolds. The phenolic OH reacts cleanly with acyl or alkyl chlorides, forming esters or ethers for use in medicinal chemistry lead optimization. Electrophilic aromatic substitution slows, given the electron-withdrawing nature of fluorines, but directed ortho-metalation—even with a lithium base—moves forward with a bit of patience and experience. In pharmaceutical routes, these aspects let chemists build up analogs of bioactive molecules with improved metabolic profiles or altered selectivity.
Synonyms & Product Names
Chemists love their alternate names. 2,3-Difluorophenol answers to a handful of synonyms, including 2,3-DFP, o,o’-Difluorophenol, and 2,3-difluoro-1-hydroxybenzene. Regulatory filings sometimes list it by CAS number 2713-33-9—a label recognized across both academic literature and commercial supply chains. Multinational suppliers might give it product codes or trade names, but purity and batch data stick as the main identifying features when researchers place orders.
Safety & Operational Standards
Handling fluorinated aromatics, especially those with phenolic groups, calls for careful discipline. Vapors and accidental skin contact can trigger irritation, so fume hoods, chemical-resistant gloves, and goggles sit at the heart of every standard operating procedure. Inhalation remains a risk due to the low vapor pressure, so splash-proof lab coats and proper airflow help limit exposure. Some users forget that minor spills can leave traces hard to clean—so surface liners and absorbents are a must. Storage protocols rely on secondary containment, away from bases and acids, since decomposition or unplanned reactions generate toxic byproducts. Emergency washes, occupational health records, and periodic retraining all play a role—not just compliance checkboxes, but real habits that keep teams safe.
Application Area
The impact of 2,3-difluorophenol rings loudest in small-molecule discovery labs and R&D departments within pharma and specialty materials. Adding difluoro motifs can stop metabolic enzymes from degrading compounds too fast, extend half-life, and change the way drugs interact with protein binding sites. Crop protection chemists use these phenolic intermediates to generate more selective, longer-lasting agrochemicals. In advanced materials, its ability to introduce new hydrogen-bonding patterns and aromatic stacking makes it attractive for specialty polymers. Less visibly, it appears in monomer synthesis for optoelectronics, pushing the boundaries of what OLED and sensor components can do. Demand flexes with innovation cycles in these industries, but every new patent or clinical candidate with aryl fluorines nudges up global need.
Research & Development
A drive for greener, cheaper, and safer methods shapes almost every R&D project involving 2,3-difluorophenol. Chemists probe new catalytic pathways, often leaning on palladium or nickel to cut down both waste and reaction times. Enzyme-catalyzed transformations linger on the horizon, promising routes that avoid toxic reagents. Analytical chemists work on finding more robust ways to detect even trace impurities, since the presence of mono- or tetrafluorophenol byproducts can alter in vitro test results. Computational chemists model the electron clouds and predict novel interactions with drug targets or materials. The research pipeline now stretches beyond pharma: new uses in batteries, solar cells, and coatings get consideration. These discoveries rarely hit the mass market overnight—each relies on years of validation and regulatory review before seeing practical adoption.
Toxicity Research
No one should assume that adding fluorines automatically makes a compound safer or friendlier to the environment. Toxicologists report that 2,3-difluorophenol, like other fluorinated aromatics, shows potential for both acute and chronic effects—often linked to oxidative stress or disruption of metabolic processes. Animal studies reveal low to moderate toxicity when inhaled or absorbed in high doses, but its precise long-term impact on organ systems and the environment remains under investigation. Persistence and bioaccumulation stir growing debate among environmental scientists. Waste handling protocols avoid mixing with halogenated waste streams or heavy metals, and incineration remains the fate of much of the resulting lab and industrial waste. Research now focuses on finding safer analogs or degradation pathways that break down products to less harmful intermediates without releasing free fluorine.
Future Prospects
The next decade promises even greater roles for 2,3-difluorophenol and its derivatives as industry seeks more robust, targeted compounds. Synthetic methods will likely shift further toward milder, high-yielding reactions that cut both energy use and hazardous byproducts. Biotransformation under engineered microbial enzymes may allow for large-scale production that aligns with circular chemistry principles. Regulatory agencies continue to tighten standards for both worker exposure and environmental discharge, so compliance drives new investments in contained reactors and digital monitoring systems. Drug designers keep chasing new fluorinated scaffolds that dodge metabolic breakdown while minimizing off-target risks. Material scientists scout for new ways to fold these phenolic building blocks into polymers with custom electronic or mechanical properties. The frontier lies in striking a balance—not just maximizing utility but also guaranteeing safety, scalability, and sustainability every step along the way.
Looking Up Close: 2,3-Difluorophenol’s Formula
Most folks rarely think about the compounds tucked away in bottles in research labs. 2,3-Difluorophenol provides a good example of how structured molecules shape daily life in unseen ways. Its chemical formula, C6H4F2O, spells out the story: six carbon atoms, four hydrogens, two fluorine atoms, and one oxygen. It might not sound like much, but each number matters to those trying to puzzle out how new materials or medicines get made.
Why Molecular Structure Counts
This formula tells more than a count; it reveals a map of connections. Imagine taking a standard phenol ring—essentially a benzene ring bonded to a hydroxyl group—and tacking on fluorine atoms at the 2 and 3 spots on the ring. It’s these tweaks that often decide everything from melting points to reactivity in the lab. Having those fluorines next to each other changes the flavor of the compound completely. I’ve found in lab work that switching a single atom can transform what a chemical does inside a reaction, sometimes flipping something from stable to explosive or vice versa.
Where 2,3-Difluorophenol Splashes In
This molecule shows up as a tool and a building block. Many chemists depend on fluorinated phenols when crafting new pharmaceuticals, pesticides, or specialty polymers. Fluorine does more than just swap out hydrogen. It shifts acidity, stability, resistance to breakdown, and persistence in the environment. For medicine, these shifts help drugs last longer in the body, sometimes making treatments more effective or predictable—which patients and doctors both want.
I’ve seen how these compounds, at just a few parts per million, can help scientists label molecules for tracking inside living cells or tweak larger molecules so they dissolve better in water or fat. That doesn’t mean every new chemical is a win. Sometimes, adding extra fluorine makes it harder to break down, raising questions for waste treatment and environmental impact. The world learned lessons the hard way from persistent pollutants like PFAS—also subtle tweaks on the basic carbon-fluorine theme.
Safety, Health, and Responsibility
Safety takes center stage in any permanent relationship with fluorinated chemicals. These compounds, including 2,3-Difluorophenol, resist breakdown. That’s a boon if you want stability, but a challenge if the compound escapes into water or soil. Handling them demands sharp attention to proper containment, ventilation, and disposal. I remember training new lab techs on the basics: gloves, goggles, lab coats, and strict procedures so no one underestimates what a clear liquid can do.
Organizations like the Environmental Protection Agency keep a close watch for good reason. Companies that create or use these molecules benefit from following updated guidelines, using advanced filtration or destruction methods for waste, and investing in research on safer alternatives. Mistakes here ripple far and wide, so regular audits and transparent reporting matter just as much as clever chemistry.
Moving Forward with Care
Science keeps evolving. 2,3-Difluorophenol, with formula C6H4F2O, reminds anyone tinkering with molecules that every small change brings a chance for discovery—and risk. Staying curious, asking questions, and learning from each trial keeps progress safe and meaningful. The tight dance of innovation relies on knowing chemicals as more than just numbers; it’s about responsibility to people and the planet.
Opening the Door to Modern Chemistry
Step into any modern chemistry lab and you’ll find 2,3-Difluorophenol putting in some serious work. This compound punches above its weight, especially for such a small, colorless liquid. Years ago, I shadowed a pharmaceutical chemist who showed me just how valuable a few tweaks to a benzene ring could be. Adding two fluorine atoms onto phenol may seem like a minor modification, but it unlocks a host of handy applications.
Pharmaceutical Building Block
For drug makers, adding fluorine isn’t just about chemical flair — it changes how molecules behave in the body. Drugs built with fluorinated pieces last longer and resist breaking down too fast in the liver. 2,3-Difluorophenol serves as a starting point for new antibiotics, antiviral agents, and central nervous system drugs. A colleague of mine working in drug development mentioned how hard it can be to get the right balance between stability and activity. Swapping in a fluorinated phenol ring turned out to be the key for one of their promising cancer therapies.
The stakes are high; according to the FDA, over 20% of pharmaceuticals approved in recent years have at least one fluorine atom in their structure. The numbers speak for themselves. Subtle changes lead to big results in the clinic, and 2,3-Difluorophenol brings flexibility to the process.
Crop Protection and Agrochemicals
Farmers lean heavily on chemists for better crop yields. As pests and diseases adapt, companies keep searching for new crop protection agents with staying power. Fluorinated phenols serve as anchors in these pesticides and herbicides. 2,3-Difluorophenol helps new products stick around long enough to be effective, cutting down the number of applications farmers need.
I once spoke with someone at a seed company who explained how the industry balances environmental safety with high crop yields. Using a compound like 2,3-Difluorophenol means stronger, more targeted protection, so less chemical runoff hits local water sources. It’s a step forward for both farmers and communities.
Expanding the Reach Beyond the Lab
Lab researchers also count on 2,3-Difluorophenol when building electronic materials. Flexible displays and sensors use organic molecules tuned for special electrical properties. Introducing fluorine atoms in the right places can boost heat resistance and improve charge movement. Companies working with OLED displays or next-generation batteries are always chasing small gains, and these tweaks often make the difference between a working prototype and a shelf-ready product.
Building Blocks for the Future
Every few years, demand for new specialty chemicals grows. Advanced materials researchers keep experimenting with new mixtures to create fire-resistant fabrics or improved industrial coatings. 2,3-Difluorophenol offers a launch pad for building customized molecules that stand up to harsh conditions or deliver longer-lasting results.
Its uses keep branching out as chemists look for more efficient, environmentally-friendly approaches. Sustainable synthesis has become more important: less waste, fewer harsh reagents, and energy-saving methods. Chemists working on green chemistry approaches turn to 2,3-Difluorophenol as a trusted foundation for innovation.
Unpacking the Numbers Behind 2,3-Difluorophenol
On paper, molecular weight looks like nothing more than a number with a few decimals. In practice, even one decimal point can spell the difference between a successful synthesis and hours wasted in the lab. With 2,3-Difluorophenol, this weight is 132.10 grams per mole. That calculation takes the following: two fluorine (F) atoms, each at 18.998, six carbon (C) at 12.011, one oxygen (O) at 15.999, and five hydrogen (H) at 1.008. Add them up and you reach the neat mark of 132.10 g/mol. It might not seem like much, but this detail underpins a lot of research and industry work.
People in labs—both in academic settings and in chemical manufacturing—make a habit of checking these numbers. Miss it by even a small margin and later steps can snowball into errors. I remember puzzling over yields in a university lab, only to find the problem came from a tiny oversight with molar weights. Since then, I double-check every input. Even experienced chemists know that accuracy with numbers like these keeps their work grounded. Mistakes at this stage ripple out, costing both time and money.
Why Care About the Details?
Even outside the lab, the molecular weight has ripple effects. For example, drug design depends on precise calculations to ensure the right dosage and to predict how a candidate moves through the body. In the case of fluorinated phenols like 2,3-Difluorophenol, small changes in mass tweak both the boiling point and the behavior inside living systems. That means chemists can't fudge the details—they have to rely on trustworthy data.
2,3-Difluorophenol itself pops up in a range of studies, often serving as a intermediate in the production of pharmaceuticals or specialty chemicals. Fluorination changes how molecules bind to targets in biological systems and sometimes boosts their stability, which in turn shapes how useful the final compound becomes for real-world use. Anyone scaling up projects from a tiny flask to a pilot plant knows that inaccurate molecular weights can turn careful strategy into waste, either by running short on materials or by producing disappointing results. I've seen production lines stall because the numbers didn’t match, which quickly leads to budget overruns and headaches.
Improving Accuracy and Trust
Fixing these issues means more than just double-checking a calculator. Open databases and peer-reviewed data, like those provided by PubChem or trusted chemical suppliers, let researchers validate their math. Many industries also cross-check with batch analysis using precise instruments for mass spectrometry. This critical step is rarely skipped—smart chemists have all learned, sometimes the hard way, that firsthand verification beats blind trust in catalogs. Consistent, transparent reporting standards help anyone from a university student to a seasoned manufacturing specialist keep work on track and compliant with regulations.
Chemistry teachers emphasize getting these basics right, not just for grades, but because the cost of mistakes jumps fast in the real world. Molecular weight—like the 132.10 g/mol for 2,3-Difluorophenol—might look small, but it keeps the whole process running smoothly. Reliable data saves money, keeps people safe, and builds trust between collaborators and clients alike. In the end, science advances best when the small details line up.
Respecting Chemical Hazards in Real-World Labs
2,3-Difluorophenol looks harmless at first. It’s a common intermediate in making pharmaceuticals and agrochemicals, but it brings its own set of dangers. Anyone who’s worked with fluorinated aromatics knows the mix of curiosity and caution—this compound isn’t just another bottle on the shelf. Having spent years in university and contract labs, I’ve seen how quick shortcuts lead to trouble, especially with liquids like these.
Personal Protection Makes a Difference
Skin contact with 2,3-Difluorophenol irritates, and even a drop can cause an uncomfortable, burning sensation. Regular gloves melt too fast. I stick to nitrile or butyl rubber gloves and change them frequently. Whenever someone came in with a splash on a cotton lab coat, it never turned out well—fibers soak up the compound, and washing it out proves tough. Lab coats made of synthetic materials resist it more effectively. Leave the sandals at home; closed shoes are a must, since phenols travel quickly through exposed skin.
Ventilation and Storage
Working with open bottles of any phenol stings the nose and throat. Good ventilation does more than keep the air clear; it protects the lungs and nervous system. At my old lab, we used chemical fume hoods with solid airflow, and we checked the airflow monitor every time. A well-sealed container with a screw-top lid keeps vapor leaks in check. These bottles demand dark, dry storage—heat and sunlight eat away at stability, and the substance can react with air if left unchecked for too long. Once, someone juggled the storage order, and degraded product ruined three days’ worth of work.
Spill Response Favors Speed and Simplicity
Every chemist spills sooner or later. Once, a small bottle rolled off the bench, and we learned the hard way that waiting wastes time. Granular absorbents soak up 2,3-Difluorophenol well. Paper towels spread it around, so I avoid them entirely. Wearing proper gloves, I go in with clay-based absorbents, then scoop everything into a sealed bag for hazardous waste pickup. A backup eyewash station saved my colleague from a more serious injury, since even a hint of this compound in the eyes needs fast, thorough flushing.
Why Training and Preparation Matter
New workers sometimes downplay the warnings on a label, but phenolic compounds teach hard lessons. Regular safety drills, easy-to-read Safety Data Sheets, and hands-on training keep everyone honest. Teams need to know where to find spill kits, gloves, and respirators. I still remember a morning spent frantically searching for goggles while a small bottle leaked—we updated our protocols that afternoon, keeping gear both visible and accessible.
Waste Disposal: Following the Rules Pays Off
Dumping 2,3-Difluorophenol down the drain guarantees visits from the environmental health office. Phenol residues pollute water sources, harm aquatic life, and break waste law. Label all waste clearly, use the right container, and schedule regular pick-ups from certified handlers. As someone who’s argued with building managers over hazardous waste lockers, I can say avoiding those fines keeps the doors open and the neighbors happy.
Building a Safer Workspace, One Habit at a Time
Chemistry never grants do-overs. Simple habits—proper labeling, closed shoes, working hoods—build a safety culture people trust. 2,3-Difluorophenol won’t respect inattention but responds well to vigilance and care. Every injury I’ve witnessed came from skipping steps or ignoring the little things. Small changes, done consistently, make a huge difference.
Why Some Chemicals Aren’t on Every Shelf
A friend once joked that you can order just about anything online, from farm-fresh eggs to rare coins. Walk into your typical hardware store, ask for 2,3-Difluorophenol, and you’ll most likely get a puzzled look. This isn’t the sort of chemical you’ll find at Home Depot or nestled between cleaning products in your local supermarket.
2,3-Difluorophenol sees most of its use in research. It plays a role in pharmaceutical development, organic synthesis, and sometimes in making specialty polymers. Handling it calls for strict safety procedures–skin contact, vapor inhalation, and spills can present health risks. These facts shape how companies sell and ship the product.
Where Industry and Safety Rule the Purchase
Over the years, I’ve seen more labs turn to chemical suppliers with robust credentials. Vendors like Sigma-Aldrich or Thermo Fisher Scientific often come up in conversations because they require paperwork and only supply registered entities. Even then, researchers face identity checks to prevent diversion. I remember a time a university required three different forms before an order could be approved.
Common search engines list suppliers, but the real transaction moves behind account verification and authorization screens. I’ve spoken with procurement folks who say even research assistants can’t place orders–one misstep creates piles of compliance headaches. Typical buyers set up institutional accounts, provide business or university addresses, and share proof of legitimate use. These checks might seem like hurdles, but after reading stories of accidental misuse and environmental contamination, it’s clear why suppliers set guardrails.
Don’t Ignore the Legal Paperwork
Most countries take chemical safety seriously. U.S. buyers deal with the Department of Transportation and Environmental Protection Agency regulations. Europe uses frameworks like REACH. Even a mistake in documenting the intended use can land an institute in regulatory trouble. Reading material safety data sheets (MSDS) before buying protects everyone working near the compound and ensures storage meets fire safety codes.
In my own university experience, we had audits on chemical inventories every quarter. Non-compliance led to lectures from the safety officer, which no one enjoyed. The rules weren’t just bureaucratic—they prevented thousands of dollars in fines and kept both new and seasoned researchers healthy.
Potential Solutions for Safe and Legal Purchase
Individuals outside academic or industrial research aren’t likely to get easy access to 2,3-Difluorophenol. That can be frustrating if you’re tackling a hobby project, but there’s good reason. One workaround involves reaching out to a certified lab or university to collaborate on the research. Partnerships often open doors for access while maintaining oversight.
For institutions, building a relationship with reputable chemical vendors streamlines the buying process. Batch ordering and a well-documented inventory system save time, too. An effective procurement policy should include staff training, review of new supplier credentials each year, and secure chemical storage.
2,3-Difluorophenol sits on the shelf in many research facilities because responsible teams do their homework. Buying it ethically and legally often means a few extra steps, but those steps protect more than just the lab–they support everyone in the chain, from scientist to supplier to neighbor down the street.