2,3,5,6-Tetrafluorobenzyl Alcohol: Evolution, Properties, and Application
Historical Development
The story of 2,3,5,6-tetrafluorobenzyl alcohol traces back to the broader world of aromatic fluorination research in the late twentieth century. Early chemists experimented with selective fluorination, chasing after compounds that could withstand heat, aggressive chemicals, and still keep a reactive handle. The notion of modifying benzyl alcohol with multiple fluorine atoms came up as electronics and pharmaceutical industries demanded finer-tuned molecules. Production scaled up through decades of refining techniques for introducing multiple fluorine atoms to aromatic rings. Researchers saw promise in the way fluorination shifted physical and chemical behavior, which led to more niche compounds getting synthesized and characterized.
Product Overview
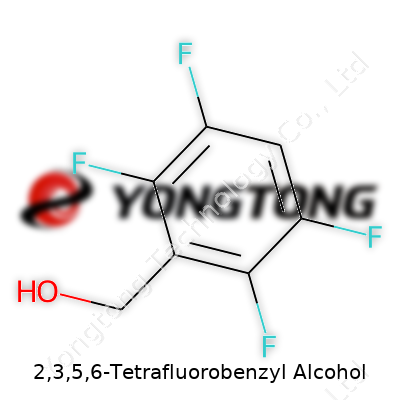
2,3,5,6-Tetrafluorobenzyl alcohol stands out as an intermediate for those building more complex chemical architectures. Its structure—a benzene ring hosting fluorines at four positions, with a benzylic alcohol—offers both stability and a foothold for further transformations. Bulk suppliers package it as a distilled liquid, usually with a purity high enough to suit research standards and some specialized manufacturing.
Physical & Chemical Properties
The colorless liquid form shows moderate volatility at room temperature and emits a distinct, sharp odor common to aromatic alcohols. The addition of four fluorine atoms significantly lowers its solubility in water but helps it dissolve in a range of organic solvents. The alcohol group, although less reactive than in typical benzyl alcohol due to electronic effects from fluorination, still allows for multiple chemical transformations. Thermal stability gets a boost, and resistance to oxidative degradation increases. Methanol, dichloromethane, and tetrahydrofuran tend to dissolve it readily in the lab.
Technical Specifications & Labeling
Bottles come labeled with chemical formula C7H4F4O, a molecular weight near 180.1 g/mol, and often a CAS number for regulatory tracking. Purity percentages and batch numbers arrive as standard. Hazard symbols for eye and skin irritants are standard features. Data sheets point out handling precautions and disposal procedures in line with local chemical safety codes.
Preparation Method
Commercial batches usually begin with tetrafluorobenzene as the precursor. A Friedel–Crafts alkylation, sometimes with a selective oxidation step, brings in the benzylic alcohol group. Lab-scale syntheses sometimes use lithium-halogen exchange to introduce side chains before oxidation. Catalysts play a role in favoring high yields while keeping byproducts to a minimum. Some groups have refined electrochemical fluorination to generate starting materials for further modification, but most processes cling to more traditional organometallic strategies due to cost and scalability.
Chemical Reactions & Modifications
Chemists often use this alcohol as a functional handle—turning it into ethers, esters, or oxidizing it to aldehyde forms. Nucleophilic substitutions occur less readily on the ring due to the electronic “shielding” from multiple fluorines, but that quality makes derivatives more selective during targeted reactions. The alcohol group works well for coupling to larger frameworks or for introducing targeted labels through protection-deprotection strategies. Hydrogenation, halogenation, and oxidation reactions proceed smoothly under carefully chosen conditions. It’s a reliable anchor in multi-step syntheses, particularly where electron-deficient aromatics are vital.
Synonyms & Product Names
Chemical catalogs and trade literature list it as TFB Alcohol, 2,3,5,6-tetrafluorophenylmethanol, and 2,3,5,6-tetrafluorobenzylol among others. Researchers sometimes shorten it to TFBzOH. In product pipelines, you may find it under proprietary labels, especially in specialty reagent offerings meant for pharmaceutical or materials science applications.
Safety & Operational Standards
The fluorinated aromatic structure brings added toxicity concerns compared to plain benzyl alcohol. Labs require fume hoods when weighing or transferring the liquid, and gloves, goggles, and chemical-resistant aprons form the minimum protection kit. MSDS sheets caution against inhaling its vapors or letting it contact skin. Waste streams must be segregated and disposed according to halogenated organic protocol. Cooling and ventilation systems cut down on fume accumulation. Training in organofluorine chemistry often precedes hands-on work, as spills can linger and residues resist easy cleanup.
Application Area
Pharmaceutical synthesis picks up this compound for introducing aryl-fluorinated motifs into drug candidates—boosting metabolic stability and changing protein binding patterns. Specialty polymers rely on it as an initiator or monomer component, benefiting from chemical resistance and modified dielectric properties. Fluorinated benzyl alcohol finds use in agrochemical research, sometimes entering the mix in pesticide and fungicide intermediate development. Analytical chemistry sees it as a tag or derivatization agent in mass spectrometry. Battery materials developers and electronics researchers turn to it for small-batch prototyping of next-generation components.
Research & Development
Academic circles tinker with improved synthesis methods that reduce hazardous byproducts or avoid rare catalysts. High-throughput screenings test how the fluorinated backbone affects bioactivity or resistance to enzymatic breakdown. Industrial researchers look at new hydrogen-bonding patterns and interactions, with the goal of crafting more hydrophobic or selective materials. Conferences regularly feature talks on next-generation fluorinated building blocks, and this compound often comes up in the context of patent literature around new synthetic strategies or compositional breakdowns of hybrid materials.
Toxicity Research
Animal models and cell lines have shown mild-to-moderate toxicity, with primary effects involving liver enzyme changes and mild cytotoxicity at higher concentrations. Chronic exposure studies are less common, but the compound sits with other fluorinated aromatics in the “handled with care” category. Researchers work to identify safe exposure thresholds, refining personal protective protocols. Analytical labs use advanced detection techniques to track traces in waste and work areas. Some early reports hint at environmental persistence once released, so disposal streams undergo regulatory review.
Future Prospects
As demand for resilient, high-performance materials and drug components rises, 2,3,5,6-tetrafluorobenzyl alcohol holds promise for more cost-effective, environmentally conscious synthesis methods. Researchers explore green chemistry alternatives that rely less on hazardous reagents. Automated process optimization can help bring down waste and energy requirements for bulk manufacture. Material scientists eye it for crafting components with unprecedented resistance to oxidation, UV, and radical attack. In pharma, ongoing studies probe new derivatization strategies that harness its electron-withdrawing nature for more targeted, less toxic therapeutic agents. Regulatory trends shift toward stricter limits on halogenated waste emissions, encouraging a wave of innovation in containment, recycling, and safer, cleaner process design.
Used in the Real World
My years in the lab taught me to respect chemicals with unassuming names. 2,3,5,6-Tetrafluorobenzyl alcohol is one of those ingredients few folks outside chemistry have heard about. The chemical itself is a mouthful, sure, but its story reveals how modern science leans on small building blocks to make big change. This isn’t just some academic compound, buried in textbooks and left to gather dust. It serves real functions in research and manufacturing that ripple out into everyday life, from medicine to agriculture.
What It Brings to Chemistry
At its core, 2,3,5,6-Tetrafluorobenzyl alcohol serves as a building block. The special arrangement of fluorine on its benzyl ring gives it a punch when mixed into larger molecules. Chemists count on that stability. The alcohol group attached means it hooks up with other chemical partners pretty reliably, which streamlines many synthetic routes. The value here isn’t just about making things work; it’s about saving time and boosting yield. In the lab, time matters and wasted batches mean lost money. Reliable intermediates like this let researchers scale up from test tubes to larger production without so many surprises.
Role in Making Pharmaceuticals
Drug companies face tough challenges: design new treatments faster, without blowing the budget. Here’s where this compound steps up. It’s often used to make intermediates in active pharmaceutical ingredients. That doesn’t sound flashy on its own, but medicines like antiviral drugs and cancer treatments don’t appear overnight. Each step in the process matters. The presence of four fluorine atoms brings strong electron-withdrawing qualities, which can change how molecules interact in the body. That means the difference between a medicine that’s potent and one that gets broken down too quickly. Top research teams have documented how introducing fluorinated units like this helps improve drug activity and absorption. So, for both the scientists and the patients waiting at the other end, these small chemical changes make an outsized difference.
Impact on Agrochemicals and Materials
Fields and factories both see the benefits. Crop protection agents—herbicides, fungicides, and insecticides—often use fluorinated intermediates to boost environmental stability and effectiveness. Chemicals with similar frameworks curb pests without breaking down before they do their job. The same principles apply in the world of specialty plastics and coatings. Materials built with blocks like this often resist heat, harsh cleaners, and sunlight better than their old-fashioned counterparts. In some cases, it even makes electronics more reliable, since certain microchips depend on precisely engineered chemical substrates.
Balancing Progress with Responsibility
Using chemicals with heavy fluorine content always requires care. Growing up around lakes and woods, I’ve seen what happens when chemicals wash off and end up where they don’t belong. Scientific papers and regulatory agencies have called for tighter control, since fluorinated residues can stick around in the environment for the long haul. Responsible handling, recycling, and waste management aren’t optional. It’s on industry leaders and researchers to keep finding safer, greener ways to create and use these compounds. Push for stricter safety checks and invest in cleaner synthesis methods. That way, cutting-edge chemistry doesn’t come with hidden costs for the planet.
Looking Ahead
Every breakthrough has a backstory full of unsung tools. 2,3,5,6-Tetrafluorobenzyl alcohol plays one of those roles, powering progress behind the scenes in medicine, agriculture, and advanced materials. Its journey from lab bench to practical solution shows what careful chemical design can accomplish—if we stay focused on the details and don’t lose sight of the bigger responsibilities tied to our tools.
Getting the Basics Right
Chemistry doesn’t always need to feel intimidating. Take 2,3,5,6-Tetrafluorobenzyl Alcohol. Pull apart that mouthful of a name, and you find a benzene ring, where four of the classic hydrogen atoms bow out, replaced by fluorine at the 2, 3, 5, and 6 spots. Throw a “benzyl alcohol” appendage onto that, and you get a –CH2OH group sitting on the ring. Count it up: six carbons, four fluorines, one methylene carbon, and a little extra oxygen and hydrogen. The math rolls out to C7H4F4O.
Molecular Weight: Why It Matters
If you ever tried to figure out how much of a compound you need for a reaction, you know why the molecular weight matters. Scientists, researchers, and even chemists in the flavor or pharma world pull this number daily. For 2,3,5,6-Tetrafluorobenzyl Alcohol, let's break it down:
- Carbon: 12.01 g/mol × 7 = 84.07
- Hydrogen: 1.01 g/mol × 4 = 4.04
- Fluorine: 19.00 g/mol × 4 = 76.00
- Oxygen: 16.00 g/mol × 1 = 16.00
Why the Structure Stands Out
Adding four fluorines to a benzene ring flips the script on what you expect from plain old benzyl alcohol. Fluorine bends chemical behavior in ways few other atoms do. Anyone handling this compound tends to notice lower reactivity on the ring—the electron pull of those fluorines throws a wrench in attempts at classic electrophilic substitutions. That’s not just textbook stuff. Try running a reaction on this molecule, and you find out pretty fast.
Real-World Relevance
Chemicals like 2,3,5,6-Tetrafluorobenzyl Alcohol don’t get as much limelight as pharmaceutical blockbusters, but they touch all sorts of research corners. Designing drugs, making specialized agrochemicals, or even developing new polymers—these steps rely on fine-tuned building blocks. By knowing the formula and weight, workers avoid costly mistakes. Small miscalculations cascade into waste or failed experiments, something any lab budget feels very quickly.
Facing Supply and Handling Challenges
Synthetic organofluorine compounds carry their own quirks. These aren’t the kind of alcohols you leave out on the bench. Storage needs careful thought—less risk than some volatile organics, but safety matters more in research worth doing right. Any spill can snarl up equipment and ruin analyses. Industry folks bring this up in seminars all the time: too many near-misses from treating specialty compounds like everyday solvents.
Working Toward Better Access and Safety
Researchers and chemical producers push for better databases and smarter labeling. Nobody likes poring through technical sheets trying to confirm a molecular weight. Standardized digital records ease the day-to-day, flagging hazards, supply limits, and preparing for scale-up. Lately, calls for greener chemistry mean extra eyes check how these fluorinated compounds degrade and persist. Vigilance in logistics and waste handling keeps accidents at bay and cuts hidden costs.
Science Grounded in Details
The specifics of molecular formula and weight ground the big-picture science. One missing digit on a calculation can waste weeks, whether the goal is a journal publication or process development for a company. Even familiar chemical territory hides traps for those who treat each compound as just “another alcohol.” It pays to remember: details shape safe, effective, and innovative science, every step of the way.
Spotting Hazards: More Than A Label
In chemistry labs, people often think of risk in black and white: hazardous or not. Something about the sound of “2,3,5,6-Tetrafluorobenzyl Alcohol” just feels intimidating for anyone without safety goggles. Yet, real hazard draws from more than its tongue-twister of a name. Getting some facts straight, this compound pops up mostly in the manufacture of specialty chemicals, and often flies under the radar compared to more notorious solvents or reagents.
Direct Exposure: The Real-world Risks
Many chemists get familiar with the risks of solvents like toluene, acetone, or even ethyl acetate. Not so many work with 2,3,5,6-Tetrafluorobenzyl Alcohol every day. The Material Safety Data Sheet (MSDS) gives the basics: it can irritate eyes, skin, or the respiratory tract with enough exposure. Think of accidental spills or splashes—those trigger the same type of stinging reaction as other benzyl alcohols, but the added fluorine atoms sometimes raise eyebrows. Fluorinated compounds don’t always behave like their hydrogen-only cousins. Chemical stability hides potential for persistent bioaccumulation if someone mishandles disposal. No one wants to see persistent organic residues end up in groundwater, especially knowing how hard those are to filter out.
Handling And Storage: Real Lessons From The Lab
In my own lab work, safety comes down to habits. With 2,3,5,6-Tetrafluorobenzyl Alcohol, you wear gloves, lab coats, and protective goggles. You keep spills off your hands, and you open it under a fume hood. Fact: the stuff doesn’t evaporate as fast as the notorious volatile solvents, but that doesn’t give a free pass. Slight warming can boost vapors just enough to cause issues in a stuffy room. Someone disposed of a similar fluorinated alcohol down a sink drain once—later, the maintenance team found traces through chemical waste sensors, highlighting how quickly things escape routine checks.
What’s At Stake For Industry And The Environment?
Modern chemical plants monitor emissions and waste streams, but not every shop floor worldwide follows best practice. Even if acute toxicity sits below the bar for famous hazardous materials, long-term buildup matters. Fluorinated chemicals have drawn extra interest as persistent organic pollutants, due to their strong carbon-fluorine bonds. There’s increasing evidence of ecological impact when manufacturers ignore proper containment. Some government agencies are starting to flag fluorinated compounds for additional screening, linking chronic low-level exposure to potential bioaccumulation in aquatic systems.
Risk Reduction: Going Beyond Labels
Simple fixes start at the bench. Closed waste containers, clear labeling, and routine safety briefings go a long way. Many labs run regular audits now, not just to tick boxes but to keep people thinking about how chemicals move—from bottle to experiment to disposal. Substitution sometimes works: using a non-fluorinated alternative when possible can knock out two-thirds of the worry. Firms can support research into new filtration materials, targeting removal of fluorinated residues from wastewater. Waste treatment companies experiment with advanced oxidation processes to break down stubborn fluorinated compounds before they reach the wild.
Why Stay Vigilant?
Anyone handling chemicals gets used to minor risks, but complacency creeps in quietly. Paying real attention to how specialty chemicals move through a lab or facility can stop minor lapses turning into bigger problems. There’s never total safety, only staying a step ahead with smart handling and honest information sharing. 2,3,5,6-Tetrafluorobenzyl Alcohol isn’t the scariest chemical lurking in a storeroom, but it still deserves respect—part of a bigger effort to keep science safe for people and their environment.
Why Purity Matters in Chemistry Labs
Chemists rely on transparency and clear standards to do their work safely and successfully. Dealing with fluorinated benzyl alcohols like 2,3,5,6-Tetrafluorobenzyl Alcohol, clean inputs reduce headaches during synthesis, scale-up, or analytical runs. Impurities turn into roadblocks: side reactions, unreliable yields, or extra cleanup. A bottle with a questionable percentage on the label wastes time and can compromise larger projects.
Getting Specific with 2,3,5,6-Tetrafluorobenzyl Alcohol Purity
On most research and production sites, suppliers list the minimum assay for this compound at ≥98% by GC or HPLC, usually verified with a certificate of analysis for every batch. This number strikes a balance between cost and reliability. Often, the difference between 98% and a 99%+ “ultra-pure” grade runs up the price without real benefit for day-to-day synthetic chemistry. For fine-tuned work — especially pharmaceutical research or reference standards — some teams opt for ≥99% purity, which slices out trace side-products.
Every lab worth its salt asks for more than just an assay percentage. A certificate describing water content (using Karl Fischer titration) and residual solvents (with headspace GC) matters, too. Water presence above 0.5% or wrong solvent residues in the bottle threaten moisture-sensitive reactions. Identity confirmation by NMR or IR helps safeguard against mix-ups, especially given the family resemblance between fluorinated aromatics.
Dealing with Impurities: Real-World Experience
Problems appear quickly if the batch carries more than a smidge of unknowns. I’ve seen a set of reactions ruined by a bottle that claimed 98% purity but came with unexpected impurities. At gram scale, a thick layer of unidentified spots in the TLC slows down progress and muddles downstream purification. Teams end up spending two days on column chromatography instead of moving on to the next step.
Simple checks can prevent drama: check the supplier’s data, run an extra NMR if something smells wrong (literally or figuratively), and don’t cut corners to save a few bucks on a cheaper, dirtier batch. Reputable suppliers will share not just a spec sheet, but data showing tests for melting point, moisture content, and organic/inorganic residue — not just a one-off purity number.
Raising Standards: What Helps Laboratories?
It pays off to foster steady communication between suppliers and lab staff. Asking detailed questions before ordering, requesting analytical data, and even arranging pre-shipment samples can dodge supply chain headaches. Documentation beats assumptions, especially as regulatory agencies expect traceability in both R&D and production.
Professional communities, like the American Chemical Society, help by setting voluntary guidelines for reagent purity. Their benchmarks have pushed chemical providers to raise their own standards and publish clear specifications. These benchmarks echo across paperwork and purchasing portals, giving researchers something firm to stand on.
Everyone benefits from an open approach to purity: fewer surprises in the flask and a sturdy backbone for reproducible science. When the focus stays on thorough verification, both scientific progress and safety outcomes stay on track.
Understanding the Chemical
2,3,5,6-Tetrafluorobenzyl Alcohol belongs to the group of fluorinated organics. Even though it isn’t a household name, researchers and lab techs recognize it for its unique chemistry, especially in synthesis work. Working with any specialty chemical requires respect, not just for its reactivity but for the risks it quietly brings into the lab, sometimes catching even seasoned chemists off guard.
Importance of Proper Storage
You’d think a small bottle couldn’t cause much trouble, but experience proves otherwise. A coworker once left a similar fluorinated solvent loosely capped after use. By the next day, vapors filled the storage cabinet, corroding some metal clamps and stinking up the whole room. That memory sticks. The right place for 2,3,5,6-Tetrafluorobenzyl Alcohol isn’t just a matter of policy; it’s a line between business as usual and cleanup duty—or worse, exposure risks.
Temperature and Environmental Control
Above all, this compound should stay in a cool, dry place. Fluctuating temps or sunlight accelerates breakdown, which can produce unpredictable by-products. Standard practice in most labs includes using temperature-monitored cabinets, kept between 2–8°C. In my own work, I trust purpose-made flammable storage fridges to keep chemicals like this one well below ambient room temperature, free from freeze-thaw cycles that make glassware and caps brittle.
Protection from Moisture and Air
Moisture-reactive chemicals often pose subtle hazards. 2,3,5,6-Tetrafluorobenzyl Alcohol may not fizz up dramatically, but humidity inside a bottle can discolor or degrade the product over time. Dry cabinets, with regular checks for desiccant freshness, shorten this risk. From first-hand experience, storing open bottles around sinks and humidifiers just never ends well. It’s easy to spot the difference between a crystal-clear sample and one that’s picked up water—the latter never performs reliably in synthesis, and sometimes gets written off as waste.
Container Choices and Sealing
Glass remains the go-to material for storing specialty organics. Polyethylene or similar plastics can absorb or react with certain fluorinated chemicals, causing bottle warping or slow leaks. At my previous lab, glass bottles with PTFE-lined screw caps lasted for years, without any sign of breakdown. The liner offers a nice chemical barrier, important even for compounds that don’t evaporate quickly.
Seals matter. Double check caps after every use, since even a tiny crack leaks harmful vapor over time. Gloves and safety glasses always accompany any handling. Don’t underestimate routine: the best protection comes from making careful closure a reflex, not a chore.
Segregation and Labeling
Storing chemicals together because they look similar tempts fate. An old neighbor in the lab made this mistake one too many times. Grouping 2,3,5,6-Tetrafluorobenzyl Alcohol with strong acids or oxidizers brings real risk; a single accident can spread fumes or even fire. Separate shelves, clear hazard labeling, and up-to-date safety sheets near the storage area make all the difference. I always keep anything fluorinated away from anything incompatible—even if storage space runs tight.
Spill Response and Best Practices
Accidents don’t wait for managers to walk by. I began every shift checking my chemical stock for leaks or odd smells. Anyone can recognize a leak in a clear bottle, but ground-in chemical smells signal older spills or evaporation. Quick cleanup, with absorbent pads and good ventilation, prevents lingering hazards.
Training counts for more than any label. Every new tech on the team runs through safe storage routines and emergency drills. Knowledge passed on in-person—what to look for, what to avoid, how to handle the bottle—beats any printed instructions. Ask a chemist who’s dealt with a minor exotherm; proper storage and handling make the difference between a lesson and a disaster.