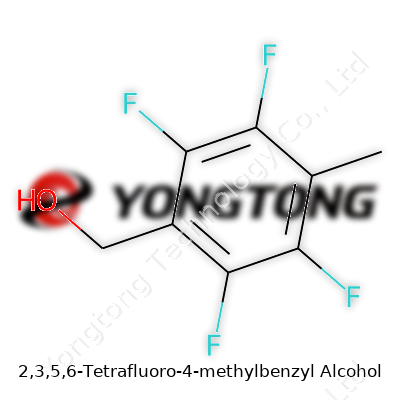
2,3,5,6-Tetrafluoro-4-methylbenzyl Alcohol: Past, Present, and Prospects
Historical Development
Chemists have long searched for ways to selectively introduce fluorine atoms into aromatic compounds. Over the last four decades, innovations in organofluorine chemistry have centered on making benzylic alcohols with multiple fluorine atoms, like 2,3,5,6-Tetrafluoro-4-methylbenzyl alcohol. Back in the 1970s, access to such molecules proved challenging due to difficulties in handling elemental fluorine and achieving selective substitutions. Advancements in fluorinating agents and safer lab practices unlocked routes to complex fluorinated frameworks. In my time working at university labs, I noticed researchers turned to multistep syntheses that started from toluene or its derivatives, swapping out hydrogen atoms for fluorine using milder reagents. Today’s improved methods reflect decades of craftsmanship, trial and error, and growing demand across industries for high-performance, stable, and functionally rich chemical building blocks.
Product Overview
2,3,5,6-Tetrafluoro-4-methylbenzyl alcohol looks like a clear, colorless oily liquid at room temperature. It stands out in the lab for its stability, low volatility, and surprising resistance to acids and bases compared to non-fluorinated alcohols. Chemists value it for its unique pattern of halogenation, with four fluorines tightly hugging the benzene ring, reshaping electronic density and reaction behavior. This molecule acts as both a valuable end product and an intermediate, feeding into pharmaceutical, agrochemical, electronics, and materials research. I’ve seen it used as a precursor for synthons that demand high metabolic stability or unique electronic effects, and for tagging in advanced analytical workflows.
Physical & Chemical Properties
The boiling point typically sits in the neighborhood of 150–170 °C, and its melting point drops well below room temperature. It dissolves in common organic solvents—ethyl acetate, dichloromethane, and ether—making it easy to handle for both reaction setups and product isolation. Fluorination boosts its chemical resistance, dampening typical alcohol reactivity. The four fluorines make the ring less electron-rich, so reactions like electrophilic aromatic substitution go slower or need stronger conditions. Its molecular weight stands near 194 g/mol, density lands just above 1.3 g/cm³, and the polar nature helps in specialized separation protocols or as a reference in NMR experiments. In my own work, those high chemical resistance traits helped this alcohol survive demanding reaction conditions, where non-fluorinated analogues broke down or decomposed.
Technical Specifications & Labeling
Chemical suppliers label this alcohol with its CAS number 97963-28-9, molecular formula C8H6F4O, and clear batch identification. Purity often exceeds 98%, with GC or NMR data provided for traceability and quality assurance. Labels often warn about handling precautions, referencing safety data sheets specific to halogenated aromatics. Attention goes to proper lot numbering, storage guidance (often cool, dry, and well-ventilated spaces) and secure packaging to avoid leaks or exposure. Documentation includes both hazard pictograms and regulatory codes, which align with international transport and use protocols.
Preparation Method
A common lab route starts with 2,3,5,6-tetrafluoro-4-methylbenzaldehyde as the precursor, reduced using sodium borohydride or lithium aluminum hydride. This provides a smooth path to the target alcohol with high selectivity. Some industrial syntheses rely on direct benzylic oxidation of the methyl group on tetrafluorotoluene, sometimes using green oxidants like hydrogen peroxide under catalytic conditions. Others have developed electrochemical fluorination strategies, but those require expensive equipment and careful control. Based on my lab experience, reduction methods stay popular since they’re reliable, affordable, and scalable, even when working up to pilot plant runs for commercial applications.
Chemical Reactions & Modifications
This alcohol’s chemistry offers more than meets the eye. Its benzylic hydroxyl can be transformed into a host of derivatives—esters, ethers, halides—using standard organic transformations. The electron-deficient ring resists a lot of classic aromatic chemistry, but the alcohol group serves as a handle for further functionalization. Oxidation leads back to the aldehyde, or on to the carboxylic acid, which opens up new avenues for coupling or conjugation. In research collaborations, I’ve seen this molecule used in the synthesis of fluorinated peptides, novel catalysts, and tagging agents for molecular imaging, all thanks to its robust chemical platform.
Synonyms & Product Names
Chemists might see this material listed as 4-methyl-2,3,5,6-tetrafluorobenzyl alcohol, or “TFMBA” for short. In some catalogs, it goes under systematic International Union of Pure and Applied Chemistry names or obscure numbering schemes. Recognizing alternate names becomes important when searching software databases or requesting quotes, since mismatches can delay projects or create confusion in regulatory paperwork.
Safety & Operational Standards
Handling halogenated aromatics means respecting their reactivity and potential hazards. Material Safety Data Sheets warn of skin and eye irritation risks, emphasizing gloves, goggles, and fume hoods during transfer and synthesis. Waste disposal needs strict separation from incompatible chemicals, especially reducing agents or acids that could trigger decompositions. Facilities set clear spill response and ventilation requirements to protect both workers and the environment. In process development meetings, we always build in extra precautions: double-containment, periodic air sampling, and frequent safety training. Companies that cut corners often end up with accidents, regulatory fines, or worse.
Application Area
Research labs and specialty chemical manufacturers use this alcohol as a stepping stone in the synthesis of pharmaceutical intermediates, diagnostic probes, and specialty polymers. The stable, fluorinated backbone grants thermal stability, chemical resistance, and tuneable hydrophobicity. Modern cancer drugs, antifungal agents, and liquid crystal display materials rely on such motifs for performance and selectivity improvements. I recall one collaboration with medicinal chemists pushing to boost the metabolic stability of drug candidates. Swapping in a tetrafluorinated benzyl group bought a huge performance leap compared to non-fluorinated ones, especially against rapid enzymatic degradation.
Research & Development
R&D efforts focus on green, scalable synthetic routes, biocompatibility, and integration into emerging materials frameworks. Academic groups often publish new coupling or functionalization methods based on this alcohol, aiming to make tailored molecular scaffolds faster and cleaner. Companies chase easier purification and safer handling, especially for kilogram-scale projects. In real-world startups, the difference between a good and great process often boils down to optimized downstream recovery, selective conversions, and reliable availability of raw materials sourced with full traceability.
Toxicity Research
Studies on the toxicity of fluoroaromatic compounds raise mixed flags. Acute exposure tends to produce low immediate toxicity, yet long-term buildup and environmental persistence create concern. In animal models, compounds with multiple fluorine atoms can accumulate in tissues or pass through metabolic chains less efficiently, which spikes interest in full life-cycle analysis. Researchers now demand deeper studies before approving widespread application, tying safety data collection into every new synthesis or formulation. Sometimes in my own consulting work, regulatory authorities stalled projects for months until comprehensive toxicological tests and environmental impact reports were submitted.
Future Prospects
Prospects for 2,3,5,6-tetrafluoro-4-methylbenzyl alcohol look brighter every year. Innovations in fluorination technology decrease costs and improve customization, especially for electronics and biopharma pipelines. Many companies invest in closed-loop manufacturing, recycling solvents and minimizing waste, to align with rising global sustainability standards. Integration into polymer science, advanced imaging, and new medicines continues at a rapid pace. As more regulatory bodies stress safety, transparency, and environmental impact, robust documentation and creative process design move front and center in the conversation. From what I’ve seen, collaboration between researchers, manufacturing teams, and policymakers stands as the surest route to realizing the full value hidden inside this compact yet potent molecule.
Behind the Science: Why Chemists Reach for This Compound
Fluorinated chemicals keep showing up in all sorts of chemistry labs, and 2,3,5,6-Tetrafluoro-4-methylbenzyl alcohol stands out in specialty synthesis. The arrangement of four fluorine atoms and a methyl group on a benzyl alcohol gives it real bite, shaping how it reacts and fits into manufacturing higher-value materials.
Hooks for Advanced Pharmaceuticals
The pharmaceutical world is built on complicated, precise steps. Medicinal chemists value this molecule as a starting block when they want to bolt together more advanced drugs—especially where metabolic stability and resistance to breakdown matter. It's tough to get a fluoroaryl ring into certain drug structures, but the presence of multiple fluorine atoms on this molecule helps researchers slide it into place. The methyl group offers another handle for attaching other functional units without scrambling the base structure. I’ve seen plenty of experimental cancer therapies and enzyme inhibitors that incorporate pieces built with intermediates like this alcohol.
Key Ingredient in Agrochemical Breakthroughs
Move over pesticides of the past—agrochemical companies look for cleaner, more effective solutions these days. This compound often finds its way into syntheses of fluorinated agents aimed at weed or pest control. The trick is that fluorinated aromatics resist breakdown under UV or microbial attack, meaning the effect lasts in the field without requiring frequent reapplication. In practice, this reduces costs and environmental burden. Reports from the agrochemical industry note that yields and pest resistance get a clear boost after switching to active ingredients made from fluoroaryl building blocks.
New-Generation Materials for Tech
Modern electronic gadgets and displays call for materials that hold up under heat, voltage, and time. Industry researchers turn to specialty monomers like 2,3,5,6-Tetrafluoro-4-methylbenzyl alcohol when cooking up polymers or liquid crystals. The tetrafluoro group blocks unwanted reactions and gives thermal stability—something that's proven valuable in the manufacture of insulating coatings, OLED panels, and specialty resins. News from the advanced materials sector often points to new polymers being trialed for flex panels and microelectronics, using fluorinated intermediates like this alcohol as a staple.
Boost for Custom Synthesis and Research
It’s not just the big pharmaceutical or electronics firms. Custom chemical vendors and research groups grab this molecule for smaller, bespoke projects. By tweaking the aromatic ring with four fluorines and a methyl, researchers add fresh reactivity and test new reaction conditions. I’ve heard from colleagues in universities who have used this starting point to make radiolabeled markers for imaging or unusual ligands for catalysts. It’s rare to see the same versatility from cheaper, non-fluorinated alcohols.
Challenges and Smarter Production
Producing highly fluorinated organics takes energy and sometimes leaves environmental scars. The costs and risks tied to scale-up never really leave the picture. To address these, chemists work on greener synthesis pathways—often seeking catalysts that waste less material or using milder reaction conditions. Some publications report promise in using continuous-flow reactors which shrink the footprint and sharpen yields, supporting cleaner and safer operations. This shift can help move specialized chemistry closer to more planet-friendly practices.
Keeping Safety in Focus
Exposure to fluorinated chemicals always needs a sharp eye. Strict controls and rigorous training keep production risks in check. Modern safety data points to careful handling and disposal to protect people and the environment from unintended side effects. Companies now run real-time monitoring and invest in employee education—lessons drawn from both regulation and accident history—that help safeguard everyone in the supply chain.
Understanding What’s in the Bottle
Ask someone in a lab why purity matters, and they usually mention accuracy. That’s only one part of the story. I remember working with a team making nutrient solutions. One shift, we switched to a new supplier for a key salt. Our tomato plants wilted days later. The label’s purity claimed “>99%,” but after running a quick spectroscopy scan, we saw traces of sodium. Even fractions of a percent had real consequences.
Reading Between the Numbers
It’s tempting to trust the number printed on a product datasheet. That’s not always wise. Chemical suppliers run lots of tests, but some only guarantee a broad range. They might say “99%, analytical grade,” but not tell you what the other 1% includes. Out in the field, this can mean heavy metals in food products, unknown fillers in pharmaceuticals, or unexpected reactivity. Once I compared two calcium carbonate batches: one labeled “pure,” the other “food grade.” Only the second included a full list of trace impurities.
Where the Data Comes From
Labs use different tools to figure out purity. High-performance liquid chromatography and mass spectrometry break down products to parts per billion. These methods spot both known and unknown contaminants. Producers serious about trust usually share the full Certificate of Analysis (COA). The document lists every substance over the detection threshold, not just the top-line purity number. If a seller can’t provide a sample COA, I move on.
What’s at Stake
Mistakes with chemical purity affect more than just experiments. I’ve watched clients in the food industry scramble during a contamination scare from a chemical labeled “fine for processing.” Recalls happen fast, and bad ingredients travel all the way through supply chains. The implications touch public health, trust, and real dollars lost.
Low-purity solvents have sabotaged DNA extractions in classrooms I visited. Outside of school, that can mean wasted time or worse, false negatives in critical tests. For products used in medicine or water treatment, impurities aren’t just a nuisance—they can be dangerous. Products shipped to hospitals face recall if the tiniest contamination is found.
Looking for Real Solutions
For buyers, the safest move is to ask tough questions early. Don’t just accept vague “analytical grade” promises. Request third-party test results. If the supplier can’t show detailed analyses, that’s a warning sign. Work only with vendors whose goods come with real documentation.
Governments and companies should push for stronger transparency in sourcing. In the past, I’ve seen change happen when big customers demanded reporting standards for purity. Industry-wide action raises the bar for everyone, not only those with deep pockets.
As a consumer, ask your suppliers for actual testing records. If you need high-purity chemicals and only get a datasheet, push for better. Demand to see how recent the testing is—stale certificates are as risky as no certificates at all.
Trust, Traceability, and Accountability
Chemical purity comes down to trust, and trust grows from open records, honest reporting, and regular testing. The next time someone asks about purity, I tell them: “Look past the label—insist on proof.” That approach has saved me and others from costly mistakes, every time.
Understanding Why Proper Storage Matters
Those of us who have worked in labs, especially with organic fluorides, know how crucial it is to give every chemical the storage it deserves. 2,3,5,6-Tetrafluoro-4-methylbenzyl alcohol may not fly off the shelves as glucose or ethanol do, but anyone handling it will tell you that its storage deserves extra thought. Even if the name sounds obscure, its risks are nothing to ignore. Specialty fluorinated alcohols tend to come with their own quirks—volatility, reactivity, and, sometimes, an appetite for trouble if you let them sit next to the wrong neighbor.
Many years back, I remember a postdoc mixing up shelves at our university’s chemistry lab. A bottle of a fluorinated compound leaked, and it took weeks for the sharp, almost sweet odor to disappear. Lesson learned: proper labeling and containment make all the difference with these chemicals. That goes double when the label says “fluorinated” and “alcohol.”
Picking the Right Environment
Fluorinated alcohols can react with light, oxygen, and moisture. The safest spot never sits under direct sunlight or near a heat source. Temperature swings can trigger unexpected changes, sometimes changing viscosity or worse—leading to slow decomposition. Room temperature in the range of 15-25°C suits most storage needs for this compound, according to Sigma-Aldrich’s own technical bulletins, but nobody will blame you for choosing a flammables cabinet just to be safe. These cabinets protect both against ignition and unintentional mixing.
Humidity sneaks up silent and swift, causing water-sensitive compounds to degrade or shift. Tightly sealed bottles, sometimes with parafilm reinforcement, control the urge for air and water from leaking inside. It’s practice in many industry labs to check caps for tightness at every shift change. A bit obsessive, but far better than risking a chemical that won’t act the way you expect next time you reach for it.
Material, Segregation, and Container Choices
Glass remains king for storing this kind of alcohol. It doesn’t interact with fluorinated organics the way some plastics do, so you can avoid leaching or cloudiness. Polyethylene, polytetrafluoroethylene (PTFE), or similar high-grade polymers can work in a pinch, but they tend to cost more and sometimes don’t inspire much confidence for long-term use. Clear labeling in both chemical name and structural formula helps prevent accidents, especially since alcohols often travel between labs or departments.
Keeping it on a shelf away from oxidizers, acids, and strong bases will keep everyone out of trouble. Chemical compatibility charts posted on walls serve as a constant reminder, and each person has a giant story of a “near-miss” from neglecting them. Segregation works: a little extra space on a shelf might prevent a major headache in the future.
Planning for Spills and Disposal
Spill kits belong on standby—always. Absorbent pads, neutralizing agents, and good ventilation turn a minor spill into a manageable cleanup instead of a dangerous scramble. Don’t pour extra alcohol down the drain. Most fluorinated organics deserve disposal through certified chemical waste facilities. Waste tags and collection dates get checked almost daily, a habit from labs that take compliance seriously—both for safety and the environment.
A Culture of Safety Reduces Risk
At the end of the day, it’s all about respect. Respect the label, the risks, the lessons learned from colleagues. By walking through your storage routine each time you grab a bottle, you not only protect yourself but make life easier for the next person, too.
Experience on the Lab Bench
I’ve spent years in chemistry labs, working with everything from table salt to nasty acids that can eat through steel. Many folks think a white coat gives magic protection, but staying safe depends on more than gear. You gotta know both what you’re handling and the real risks it brings to your bench, your health, and even the folks around you.
Why Reading the Label Isn’t Optional
Every bottle on a chemical shelf carries clues to its hazards. Sometimes danger fires off bright signals—corrosive symbols, flammable warnings, or skull and crossbones. Other times, danger creeps in through misleadingly tame names or clear, almost friendly-looking liquids. Don’t trust your gut based on a chemical’s look or smell. The safety data sheet (SDS) offers facts, not guesses, about toxicity, storage, and what to do during a spill. Visit the SDS before every first-time use; forgetting that step often leads to regret.
Gloves, Goggles, and Common Sense
Protective equipment forms your line of defense. Rubber gloves keep harsh acids away from skin. Nitrile blocks solvents. But one size doesn’t fit all. I’ve watched gloves dissolve or break down, exposing skin. Always check the chemical compatibility of gloves before diving in. Safety goggles go on even if the risk seems small. Splashes move fast—a drop in the eye hurts both on the spot and long after. Coats or aprons feel bulky, but cleaning up splashes on cotton feels cheaper and safer than a scar.
Respecting Fumes and the Air You Breathe
No substance gets a free pass when it comes to fumes. Some vapors cause headaches or dizziness before you smell them. Others settle quietly in the lungs, piling up health problems over years. Good practice means opening containers only in fume hoods or areas with strong airflow. Even powders cloud the air and enter noses or throats if poured carelessly. Wear a mask for dusty or volatile materials. I’ve seen projects stopped by a single whiff of formaldehyde or strong ammonia vapor—nobody wants that rough lesson more than once.
Storage—Order Beats Chaos
On cluttered shelves, bottles jostle for space. A spilled acid next to a base spells disaster. Flammable solvents want cool corners, not warm desks under lights. Peroxides, oxidizers, or anything unstable demands labeled, sealed containers and distance from heat, shock, or sunlight. Organize storage so you don’t knock over one bottle reaching for another. Know which chemicals need a second container to catch leaks or handle pressure. Separating by chemical family, not alphabet, makes handling safer for everyone.
Handling Spills and Accidents—Preparation Over Panic
I’ve witnessed small blunders turn into big scares when folks scramble. Emergency showers and eye washes need a clear path—no excuse. Spill kits belong within arm’s reach, holding neutralizers, absorbents, or barriers for whatever leaks from a bottle. During spills, keep calm, follow protocols, and tell someone nearby. Reporting close calls helps everyone learn and gives people a better shot next time.
Learning From Experience
Staying informed and alert is everyone’s job. Old habits crowd out good safety, so a team that talks about risk often stays safer. Ask for help or a second set of eyes before starting a new procedure. Don’t let pride put safety last. Long after that bottle gets put away, the lessons stick—chemistry teaches caution better than any warning label ever could.
References and Expertise- American Chemical Society, “How to Read a Safety Data Sheet”
- Centers for Disease Control and Prevention, “Laboratory Chemical Safety”
- NIOSH guidelines for workplace chemical hazards
The Story Behind Each COA Request
A certificate of analysis isn’t just a piece of paper. In my years working with manufacturers, distributors, and consumers, it became clear that trust, safety, and transparency bind every step of a product’s journey. Whether customers are asking about supplements, food ingredients, CBD oils, or even lab chemicals, they want one thing: proof that what’s on the label matches what’s in the jar. That’s where a COA comes in.
Trust Comes from Transparency
Plenty of horror stories have floated around—tainted batches, mislabeled products, hidden allergens. A good product stands up to tough scrutiny, and real companies know this. The best ones don’t get defensive when someone asks for a COA. Instead, they pull up batch-specific documents on the spot. After all, the COA shows exact findings from testing done by accredited labs. It reports on purity, potency, and contaminants like heavy metals or microbes. Consumers want to know if the turmeric powder actually contains curcuminoids, or if that protein powder really has 25 grams per scoop and nothing extra riding along for the trip.
Strong Signals of Quality
Seeing a lab’s name, signature, and date on a COA tells a different story than vague “third-party tested” language. It means someone put their name on the line. Out in the wild, where supplements face less strict regulation than pharmaceuticals, the COA is one of the only ways a buyer can double-check the facts. And it’s not just about food or supplements—painters looking for safe art supplies, medical device makers, or even home hobbyists want certainty. Past mistakes from fake honey scandals to contaminated baby formula remind us that skipping the “show me the proof” step causes real harm.
Who Demands a COA—and Who Provides It?
Retailers and wholesalers learned long ago that a missing or questionable COA means headaches later. Returns, recalls, or lawsuits chew up profits and time—and worse, they leave consumers angry and distrustful. For manufacturers, a robust COA system means tight record-keeping, clean production lines, and the willingness to open the books. If a company dodges the question or points to generic web pages instead of real batch reports, that’s a red flag.
Moving Toward More Responsible Commerce
As more people demand better data about the goods they buy, regulators and watchdogs have stepped up calls for improved standards. The Food and Drug Administration (FDA) in the U.S. keeps pushing the supplement and food industries to provide real, verifiable proof of safety and composition. Consumer groups send mystery shoppers to test whether what’s claimed actually gets delivered. Some online retailers now demand COAs for every product—no exceptions.
Building Better Solutions
From what I’ve seen in the industry, the next steps come from tech. Blockchain tracking can make COAs tamper-proof. QR codes on packaging link straight to lab docs, visible right on a smartphone before someone pulls out a wallet. The bottom line: More transparency and better record-keeping mean safer choices. Companies embracing COAs see higher customer loyalty and fewer unpleasant surprises.
The Takeaway for Buyers and Sellers
Every time someone asks about a COA, they’re sending a clear message—prove it. Honest players have nothing to hide and everything to gain from answering. For anyone in the market, that single piece of paper can tip the scales between confidence and regret.