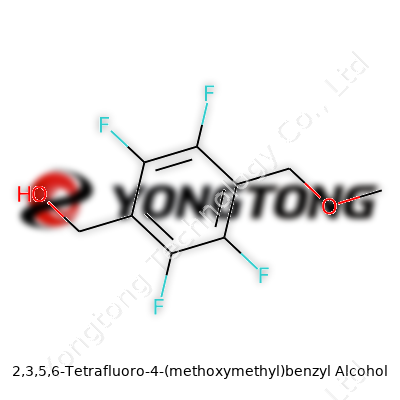
2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol: From Lab Bench Curiosity to Broad Applications
Historical Development
Researchers first encountered 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol in the wave of late-twentieth-century fluorine chemistry. Chemists in academic and industrial labs turned to fluorine not just for its headline-grabbing reactivity, but also for the performance it brings to pharmaceuticals, agrochemicals, and materials science. The synthesis of polyfluorinated benzyl alcohols did not start out as a major research target, but once pioneers noticed the distinct behaviors of tetrafluorinated rings—greater metabolic stability, enhanced lipophilicity, and altered hydrogen bonding patterns—more teams joined the push. The structure itself, with its attractively simple methoxymethyl group nestled among four stubbornly electronegative fluorines, became a building block for new ideas and downstream innovation.
Product Overview
2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol acts like a Swiss Army knife in the world of chemical intermediates. Its utility stretches from specialty polymers to drug candidates because chemists can dress it up or pare it down through a range of transformations. In my past work with functional fluorinated aromatics, I saw first-hand how this alcohol often served as a road in drug discovery projects—roaming between core structures in medchem libraries and evolving side chains for agrochemicals. The stability brought by the four fluorines lets it show up where oxygen- and nitrogen-rich environments would chew up lesser aromatics.
Physical & Chemical Properties
This compound typically presents as a colorless to light yellow viscous liquid at room temperature, settling between the nucleophilic softness of benzyl alcohols and the rugged chemical resistance of polyfluorinated rings. Its molecular weight clocks in at 224.15 g/mol, and it bears a relatively low vapor pressure, which keeps it manageable in most lab setups. The boiling point stands higher than one would expect for a standard benzyl alcohol, and a strong electron-withdrawing effect from the four fluorines leads to unique acidity and reactivity: a pKa often several units below its non-fluorinated cousins. Hydrophobicity climbs as the logP rises, yet the single –OH group lends enough hydrogen bonding to retain some solubility in polar organic solvents.
Technical Specifications & Labeling
Purity beyond 98% typically satisfies industrial and research needs, with gas chromatography and NMR spectrometry confirming the structural fidelity. Labels caution about storage away from strong acids, oxidizers, and bases, as even robust polyfluorobenzyl derivatives can unravel in harsh conditions. Strict attention to batch traceability and regulatory listing becomes routine—especially when contemplating scale-up or submission to regulatory authorities for downstream uses in drug or materials manufacturing.
Preparation Method
Production often starts with 2,3,5,6-tetrafluorotoluene, accessible by selective fluorination of toluene. Through side-chain oxidation or direct methoxymethylation—sometimes via halomethyl ethers or the use of formaldehyde and methanol under acidic catalysis—the methoxymethyl substituent attaches at the para position. Subsequent benzylic oxidation, typically using mild oxidants like manganese dioxide or silver oxide, followed by careful hydrolysis, yields the desired alcohol. Flawless exclusion of moisture and air, diligent control of temperature, and a knack for purification with column chromatography keep the final product within spec.
Chemical Reactions & Modifications
Chemists love to twist and modify polyfluorinated benzylic alcohols into new forms. Common transformations include etherification (tucking away the alcohol as an alkyl or silyl ether), acylation, and direct nucleophilic substitution at the benzylic position. In drug discovery campaigns, this alcohol forms the anchor for coupling reactions—Suzuki and Stille cross-couplings—pushing the limits of what is accessible on aromatic fluorine-rich scaffolds. In my experience, the electron-withdrawing ring encourages reactions with soft nucleophiles and resists most electrophilic attack, making it an especially sturdy handle for further modification under harsh conditions.
Synonyms & Product Names
Through years of catalog shopping and patent surveying, I have noticed this compound wears several names. Chemists searching for it by 2,3,5,6-tetrafluoro-4-(methoxymethyl)benzyl alcohol, tetrafluoromethoxymethylbenzyl alcohol, or even abbreviated as TFMMBA, will land on the same versatile substance. Catalog entries may carry supplier-specific codes, but the core identifiers and IUPAC names remain stable across the industry.
Safety & Operational Standards
Like most specialty intermediates, this alcohol demands careful handling. Protective gloves and splash-resistant eyewear are standard. Though not classed as particularly acutely toxic, the combination of high lipophilicity and chemical persistence suggests caution. Lab tests reveal low but measurable absorption through skin and possible respiratory irritation, mostly from splashes or accidental aerosol. MSDS sheets highlight precautions against prolonged or repeated contact and stress the need for well-ventilated handling spaces. As someone who has worked with similar polyfluorinated intermediates, I keep calcium gluconate gel at the ready, stay alert for any spillage, and follow triple containment strategies to minimize risks.
Application Area
Applications run deep. In pharma, the alcohol scaffolds fluorinated analogs of lead compounds—altering metabolism or boosting membrane permeability in ways non-fluorinated drugs rarely achieve. Polymer scientists chase after its chemical toughness, using its structure to impart heat or solvent resistance to niche materials like wire coatings or battery components. Agrochemical developers blend it into new fungicides and herbicides, counting on both the metabolic stability of the fluorinated ring and the functional leverage of the alcohol side chain. Some electronic materials teams use it to surface-modify semiconductors or create low-energy coatings. The variety reflects the confidence that comes with a molecule combining stubborn stability, moderate reactivity, and the strategic presence of both polar and non-polar functional groups.
Research & Development
The journey from bench to scale-up brings its own bag of problems. The main challenges revolve around improving selectivity in side-chain modification and pushing yield above the troublesome bottlenecks that usually show up near the downstream stages. Collaborative efforts, pairing the experience of industrial process chemists with university-led basic research teams, have solved some major headaches, replacing older, waste-heavy oxidations with greener, catalytic systems. A recent trend in my own R&D experience involved automating purity checks and real-time reaction monitoring, which catches impurities and prevents costly batch reworks.
Toxicity Research
Polyfluorinated organics draw more toxicologists than most new chemical entities. Early assays show that 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol has an LD50 well above threshold concern for oral and dermal exposure, but the trouble starts in chronic settings—potential for bioaccumulation lingers just as it does for PFAS relatives. Regulatory bodies and independent scientists keep the pressure on, calling for more thorough environmental breakdown studies and longitudinal assays. Persistent low-level exposure across soil and water systems could accumulate in mammals at the top of the food chain. While acute toxicity remains low, studies highlight the need for responsible disposal and treatment of waste streams.
Future Prospects
Future prospects look promising for this benzyl alcohol derivative. As green chemistry practices mature and demand for rugged performance in pharmaceuticals and materials increases, high-value intermediates like this will see broader, safer use. Research teams are exploring bio-based fluorination routes, aiming to lessen the environmental footprint from mineral fluorine sources. Advanced computational tools, including AI-driven modeling, are opening up routes to derivatives outside the standard aromatic core—reimagining what polyfluorinated benzylic alcohols can offer. If the industry focuses on sustainable manufacturing, careful stewardship, and responsible waste management, both performance and safety can step forward together.
What’s in a Chemical Structure?
Back in college, structural drawings in organic chemistry could seem like secret codes. Over time, I figured out that learning the language of chemistry took more than memorizing rules. Looking at a compound like 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl alcohol, this kind of name tells us exactly what sits where on the aromatic ring. It’s specific, balancing art and precise science.
Visualizing the Molecule
Four of the hydrogen atoms on the benzene ring have been swapped for fluorine atoms at positions 2, 3, 5, and 6. Each substitution changes how the molecule behaves. At position 4, a methoxymethyl group attaches, carrying an oxygen atom that brings up questions about reactivity and polarity. Finally, the benzyl alcohol group, a -CH2OH, anchors the arrangement, opening doors for connection in bigger molecules, like pharmaceutical candidates or advanced materials. Each group has an impact, sometimes subtle, sometimes major, steering the course of the whole structure.
Fluorine's Influence
Fluorine sits as one of the engineers of modern pharmaceuticals and agrochemicals. It’s heavier than hydrogen and pulls electrons hard, which tweaks the way bonds work across the molecule. Four fluorines on a benzene ring isn’t common — such substitution creates bulky shapes and less reactive areas. This makes the core of 2,3,5,6-tetrafluorinated aromatics tougher for regular enzymes to break down, helping drugs with fluorine hang around in the body longer or blocking soil bacteria from shattering pesticides too fast. In labs, I’ve seen how swapping even one hydrogen for fluorine can extend a compound’s effectiveness or keep it from breaking down before it finishes its job.
Methoxymethyl and Benzyl Alcohol: Practical Considerations
That methoxymethyl group tacked onto the aromatic ring adds a cloud of electron density, shifting how the molecule behaves with acids, bases, and biological targets. The oxygen gives a polar handle, often improving solubility or altering how the molecule fits into enzymes. A benzyl alcohol joins hydrophobic strength with an alcohol’s reactivity, which helps chemists modify the compound further — either to build on the structure for research or pivot to new pharmaceuticals.
Why Does This Structure Matter in Real Life?
Fluorinated aromatics aren’t just lab curiosities. They shape some of the most advanced drugs, crop protectants, and specialty chemicals out there. Complexity like this often translates into potent biological activity and a longer shelf life. If you’re formulating a medication to survive the acidic stomach and still work in the body, fluorinated rings often play a central role. In materials science, such a combination of groups resists heat and doesn’t break down under stress, which matters for coatings and electronics.
Potential and Challenge
Synthesizing heavily fluorinated aromatics isn’t easy. Those fluorines make the ring less reactive, so attaching extra groups — like methoxymethyl or benzyl alcohol — takes clever planning and the right catalysts. In industry, making these compounds safely and sustainably continues to be a challenge. Green chemistry practices push for fewer hazardous byproducts and smarter energy use. While I’ve worked with teams that stare at safety data sheets for hours, it always hits home: chemistry walks the line between risk and reward. Careful design and constant oversight keep the work safe for both chemists and the environment.
Looking Forward
The demand for finely tuned molecules, from smart drugs to resilient electronics, keeps driving careful study of chemical structures like 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl alcohol. Every atom, every bond serves a purpose, shaping tomorrow’s products bit by molecular bit. The effort poured into understanding these details pays off across medicine, materials, and our everyday lives.
Driving Progress in Construction
Whether you’re pouring the foundation for a home or patching up a weathered sidewalk, this product shows up as the reliable backbone. Contractors value its consistent strength—projects stay on track, delays drop. Road builders lean on it for smooth pavement that holds up against rain, trucks, and time. At sites across the country, you’ll spot workers mixing, pouring, shaping. Durability doesn’t just save headaches; it lowers costs over years by dodging repairs. With climate in the headlines, more teams now ask how a product like this can cut emissions and create healthier spaces for the folks who use them daily.
Supporting Modern Farming
Farmers have long faced unpredictable rains, poor soils, and the worry that a season’s work could disappear in one bad week. With this product, they get a soil booster that helps roots spread deeper, water stay available, and nutrients linger longer where crops need them. Better yield means steadier income from the land. Agriculture shifts fast—droughts grow tougher, and input prices climb. By working its way into daily farm practice, this product offers practical hope: fields hold moisture, and every drop of fertilizer counts. That’s real peace of mind when every harvest matters.
Powering Industry and Manufacturing
Walk through any busy factory, and you’ll spot this product in action. Some machines run smoother because their lubricants resist breaking down under pressure; others maintain clean surfaces thanks to the abrasive side that tackles buildup. Chemical plants tap into its ability to control pH or stabilize temperature in big batch reactions. Manufacturing moves fast, and small delays cost thousands. Firms invest in materials that do their job without fail—this product fits right in by keeping operations predictable. That reliability becomes a selling point all on its own, especially for companies supplying products that touch daily life.
Improving Everyday Living
Not every benefit shows up in a lab or report. Homeowners find this product in garden centers, tucked on hardware store shelves, or blended into paints and cleaners. Someone fixing a leak in their basement can count on it to plug cracks and hold up to damp. Others add it to backyard soil for healthier lawns or thrive thanks to indoor air filters using this ingredient to block dust and pollen. Over the years, little upgrades add up. What once seemed like a specialist’s tool now shapes how people cook, clean, and relax at home.
Addressing Community Challenges
Communities use this product after storms or during public works upgrades. Emergency crews patch up dangerous potholes with a quick-setting version. City planners count on water purification systems strong enough to filter run-off in heavy rain. Some cities look to it as a key part of new green infrastructure—helping manage stormwater and keep pollution out of rivers. By serving in so many daily roles, this product’s reach extends across communities. Public trust grows strongest with solutions that last, remaining usable long after the trucks leave.
Looking Toward Safer, Greener Solutions
Real advances don’t stay locked in academic journals. Teams of researchers, builders, and planners keep looking for ways to make this product safer and lighter on the environment. Whether by tweaking the recipe, lowering energy during production, or recycling material at the end of its life, the focus stays on protecting people and planet. Public health experts track workplace safety, neighbors join in local projects, and policy makers set higher standards. The work continues because this product shows up everywhere, shaping the way people work, eat, travel, and grow.
Understanding What Purity Means
Purity sounds simple at first—a substance is pure if nothing else sneaks in. But with most materials, especially those used in labs or manufacturing, purity carries deeper meaning. Labs chase that elusive “99.99% pure” tag, but every process introduces risks: dust, moisture in the air, traces of other chemicals from old containers. Even a quick touch with bare hands adds oils or lint. As someone who’s spent hours prepping solutions for chemistry projects, I’ve seen tiny mistakes blow up experiments. A stray grain of table salt never looks like much until it’s thrown into a test tube and spoils the batch.
Purity matters for real-world reasons. Pharmaceuticals, for example, must reach high levels of purity to keep patients safe. Any contamination—metals, leftover solvents, even pieces of plastic from pipettes—risks unexpected reactions. According to the U.S. Pharmacopeia, common drugs are rejected if impurities top even 0.1%. Labs pay for that kind of screening, not just for rules but because someone’s health is on the line.
Purity in Everyday Life
Step out of the lab, and purity becomes a familiar story. Tap water passes through massive filters and treatments, removing bacteria and metals. Your phone’s screen relies on pure silicon. Food companies test for everything from pesticide residue to pieces of insect. Here, purity measures trust. When a recall hits, it’s often from a batch losing its purity, finding salmonella or traces of lead far higher than anyone wants.
Outside the headlines, people rarely notice until something goes wrong. I remember reading about the 2008 melamine scandal in China’s baby formula. Milk, watered down to stretch supply, was laced with melamine to fake higher protein. Thousands of infants fell sick. For companies chasing the bottom line, maintaining purity turned into an afterthought, with tragic results. The lesson hangs in the air: skip the details, and someone pays the price.
How Purity Gets Protected—And Lost
So how do companies keep things pure? The answer often begins with good containers. Glass bottles, stainless steel drums, or plastic vials come sterilized and sealed. Even the choice of closure matters. Tight screw caps or vacuum seals ward off air, moisture, and anything floating around the warehouse. Pharmacies wrap pills in blister packs to fend off humidity and stray bacteria.
People working with pure substances go to great lengths, donning gloves and masks, wiping containers with alcohol, and storing sensitive powders in refrigerators or dry cabinets. Somebody once told me their electronics workshop used a filtered cleanroom just to build circuit boards—one speck of dust can take down an entire batch. Big chemical firms keep dedicated storage rooms at set temperatures, with filtered air and constant monitoring.
Problems often crop up after delivery. Packages arrive punctured, seals sit loose, or containers pick up condensation on shelves. This part shows up most in poorly funded institutions—hospital storerooms or rural clinics where boxes sit for weeks on rusty racks. Solutions mean more than just buying fancy equipment. Training matters: teaching staff what to check before scooping powder into a beaker, how to notice tampered caps, and why every spill or mistake should be logged and fixed.
Building a Culture That Values Purity
Purity stands on more than just machines and test results. Trust grows from habits, routine checks, and sharing mistakes, not hiding them. Companies can offer regular feedback sessions, clear storage rules, and better labeling. Regulators need surprise checks, open reporting systems, and harsh penalties for those who cut corners. No fancy technology saves a batch if the people running the show forget why it all matters.
Daily Risks and Common Sense Precautions
Products we use at work or home come with their own safety baggage. From cleaning powders under the sink to the spray paint in the garage, each product invites a question—how risky is it? Growing up on a small farm, I saw mishaps caused by overlooked warnings and poorly stored chemicals. That old weed killer tucked beside the fertilizer probably sat there for years, eating away at curiosity until someone touched it without gloves. Burnt fingertips and a call to the regional poison line—not the sort of drama anyone wants.
Good handling practices can prevent many small disasters. Don’t mix household chemicals, especially if you don’t recognize every ingredient. Bleach and ammonia both aim to keep things spotless, but combined, they unleash toxic gas. These mistakes don’t just hurt the person pouring them—they put the whole household at risk. It’s why proper training never goes out of style on job sites or in home garages.
Labels and Regulations Matter
Safety instructions printed on bottles sometimes look like legalese, but they matter. The U.S. Environmental Protection Agency flags solvents and pesticides with signal words like "Caution," "Warning," or "Danger." Retail stores stock everything from bug foggers to paint thinners. Each one must comply with rules set after years of studies, health incidents, and government reviews. Labels deliver the cliff notes of all that research. Skip over the fine print and you lose the benefit of decades of grim trial and error.
My own background in community science programs hammered home the importance of reading MSDS sheets (Material Safety Data Sheets). They list the harm you could face—like skin burns, lung irritation, or worse. They even offer up first-aid steps that many folks only hear about the hard way. School labs often miss the mark on teaching kids what to do if something splashes in their eyes, and it’s a lesson better learned from a page, not from panic.
The Trouble with Toxicity
Most people know to keep drain cleaner away from kids, but chronic exposures often slip through the cracks. Take common solvents, for example. Paint strippers can affect nerves, memory, and breathing if the fumes hang in the air. Farmworkers handling pesticides face long-term risks that don’t always show up right away. Years of study link these exposures with higher rates of some cancers and nerve diseases. The World Health Organization and respected journals back up these findings. We need more focus on the long game, not just protecting against accidents today but long-term health, too.
Solutions That Stick
Good ventilation keeps air moving and pushes chemical fumes outside, instead of into lungs. Locking up chemicals seems simple but works wonders, especially with kids at home. Industry groups and safety organizations offer practical guides for storage and emergency procedures. Sharing this knowledge can make a real difference—no fancy equipment required.
Switching to less toxic products makes sense when the option exists. Companies keep adding safer alternatives every year, thanks in part to watchdogs and informed customers who ask tougher questions. At work, hazard training, clear signs, and quick-access first-aid supplies do more than tick a box—they save lives.
Collective attention and steady pushback against complacency keep safety an everyday habit. Respect for warnings, smarter choices, and a willingness to share lessons learned create a safer space for everyone, both at work and at home.
Beyond a String of Numbers
Let’s start with the basics—CAS numbers. Every compound out there in the world of chemistry gets one. This is how researchers, engineers, and even factory workers keep track of what’s what in a crowded landscape. For the compound 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol, the CAS number turns out to be 886763-06-0. Seems minor until you try to order the wrong chemical, or worse, use the wrong one in your project. Small missteps like that cost time, money, and sometimes health and safety.
Why This Accuracy Matters
Diving into my own experience, I remember a project where a multi-step synthesis required meticulous tracking of intermediates. One digit off in a CAS number meant starting from scratch. The whole system thrives on each chemical identity being unique and clearly marked. Factories depend on this accuracy to keep their workplace safe. Researchers need it to publish reproducible results. With thousands of new chemicals described each year, the chances for mix-ups only grow.
CAS Numbers in Real Life
Pharmaceutical teams aren’t alone here. Try working in paints or polymers and the story’s the same. Anyone dealing with chemicals—students, surgeons, city water engineers—knows the headaches of confusing two similar-sounding names. With CAS numbers, ambiguity melts away. Data stays connected across languages and companies, regulations stay tight, and those crucial safety sheets stay precise.
In compliance checks, regulatory teams zero in on these CAS codes. A law might ban a solvent under a certain number. Substitute a chemical with a different CAS, and doors might open where they should stay closed. This cuts both ways: businesses avoid unnecessary hurdles, and communities avoid hidden dangers. Nothing kills trust faster than discovering the chemical you meant to avoid was just hiding behind a new name.
More Than Just a Label
Some folks call CAS numbers “license plates” for chemicals. Doesn’t quite describe the full story. These numbers stay attached from the first lab report to global shipment. Type 886763-06-0 into a scientific database, and details jump out: melting point, structure, regulatory status, toxicity warnings. This transparency underpins smart decisions. Chemists waste less time double-checking synonyms; industries limit liability; the public faces fewer risks.
Facing the Challenge of Chemical Cataloguing
Tracking down the right data hasn’t always been straightforward. Overlapping databases, legacy names that never quite die, new synthetic routes creating slightly tweaked molecules—each one gets its own CAS number. Without a universal system, a single conversation about safety or research direction could stall or veer off course. Instead, the CAS registry gives every player a common map.
The challenge isn’t going away. With the rise of advanced materials, biotech, and green chemistry, accurate identification keeps growing in importance. So for 2,3,5,6-Tetrafluoro-4-(methoxymethyl)benzyl Alcohol, that number—886763-06-0—offers something priceless: a single, reliable key to a whole library of knowledge.