2,3,5,6-Tetrafluoro-1,4-benzenedimethanol: A Comprehensive Look
Historical Development
The story of 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol runs alongside the broader evolution of specialty chemicals geared toward advanced materials. Chemists began exploring tetrafluorinated aromatics as early as the mid-twentieth century. Early industrial curiosity in fluorinated building blocks grew hand in hand with demand for plastics that wouldn’t break down in harsh chemical settings. It wasn’t enough to invent tough polymers—chemists required intermediates like this one to push the limits of how plastics perform in real-world applications. Tetrafluoro-1,4-benzenedimethanol’s specific popularity rose after its synthesis routes matured, driven by surging calls for fluoropolymers in electronics and coatings. Researchers saw potential and patent databases expanded quickly.
Product Overview
Chemically speaking, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol (sometimes called 2,3,5,6-TFBDM) belongs to the fluorinated aromatic alcohol family. The material appears as an off-white crystalline solid and carries two stable hydroxymethyl groups mounted on a four-fluorine substituted benzene ring. The unique arrangement delivers a rare blend of chemical resistance and reactivity, distinguishing it from traditional benzenedimethanol variants. Manufacturers today focus on keeping impurities low and trace moisture below detection limits, so it fits demanding downstream reactions.
Physical & Chemical Properties
With a molecular weight just under 222.1 g/mol, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol remains stable at room temperature. It melts in the 80-83°C range and dissolves easily in organic solvents such as dichloromethane, DMF, and DMSO. The compound doesn’t offer much solubility in water, which is expected from a heavily fluorinated aromatic core. Its structure contrasts sharply with its precursor, 1,4-benzenedimethanol, mainly because the fluorines block sites that would undergo easy oxidation or unwanted side reactions. That core stability supports longer shelf lives, particularly in sealed containers.
Technical Specifications & Labeling
High-purity batches typically carry a purity of over 98%, and analytical reports spell out content of known byproducts and metals. Labs regularly rely on NMR, FTIR, and HPLC to confirm the absence of contaminants before shipping out. Safety labels require a GHS classification because inhalation and skin contact can cause irritation. Drum and ampoule packaging must carry batch numbers, CAS identifier (17871-87-3), net weight, date of production, and storage instructions to avoid sunlight and moisture exposure.
Preparation Method
Large-scale synthesis of 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol usually starts from 2,3,5,6-tetrafluoroterephthalaldehyde or commercial hexafluorobenzene. Once chemists anchor the two hydroxymethyl groups onto the aromatic ring—often using reductive alkylation or Grignard reactions—they press for thorough purification. Recrystallization and column chromatography come into play, with solvents recirculated when possible to cut waste and cost. In practice, minor tweaks to reaction temperature, solvent mix, or base choice can swing yields by a big margin. Older routes sometimes suffered from nasty byproducts, but process improvements over decades now mean safer handling and better throughput.
Chemical Reactions & Modifications
The two -CH2OH groups on the fluorinated ring act as versatile handles, allowing conversion into ethers, esters, or varied crosslinked resins. Epoxy chemists appreciate how it can serve as a starting point for functional oligomers. Fluorine atoms on the ring itself resist nucleophilic and electrophilic substitution, which shields the product from degradation even under rough polymerization conditions. You sometimes see selective deprotection or halogen exchange at one position when specific custom derivatives are needed, but on the whole, its structure pushes back against chemical attack. This resistance extends the lifetime of materials incorporating it.
Synonyms & Product Names
Over the years, suppliers and chemists have used names like TFBM-diol, 2,3,5,6-tetrafluoro-p-xylylenedimethanol, and 2,3,5,6-tetrafluorobenzene-1,4-dimethanol for this same molecule. Each name reflects a different naming culture or downstream application. Catalog codes at different chemical shops may change, but the CAS number stands as the key identifier among regulatory databases and customs forms.
Safety & Operational Standards
Safe use starts with understanding this material’s risks. Prolonged contact or inhalation can irritate mucous membranes, so ventilated workspaces and gloves make a difference. Dust can cause eye discomfort. In the event of a spill, sweeping up without creating airborne particles beats trying to vacuum or flush the stuff. Regulations push producers to disclose all relevant hazard information through Safety Data Sheets and to train handlers on proper storage. Product waste must be disposed of as hazardous chemical waste given its persistent aromatic core and fluorinated nature; dumping it irresponsibly could contaminate ground or water sources. Plant operators check that fire systems are ready, because organic solids can feed a blaze if ignition starts, even though this compound is less flammable than lighter organics.
Application Area
Across industry, this compound finds niche roles in high-value plastics, specialized adhesives, and as a building block for fluorinated polyesters. The electronics sector uses it in dielectric coatings and as part of resist formulations for semiconductor lithography due to its ability to withstand harsh solvents and high temperatures. Protective coatings with a backbone built on this type of molecule deliver strong weather and chemical protection, extending the life of everything from aircraft parts to chemical tanks. Over time, as electronics minaturize further, fluoroaromatic components like this one become essential in insulators that won’t fail under heat or chemical assault.
Research & Development
Labs exploring new routes for energy, batteries, or flexible displays continually bump into the limits of traditional organic materials. For a while, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol held a small, but growing, share of the R&D supply chain. Work on fluorinated polyesters and specialty epoxy resins tie closely with this molecule, either as a monomer or a modifying co-monomer. Research groups probe its ability to resist hydrolytic breakdown, aiming for polymers that won’t embrittle after countless temperature or humidity cycles. Collaborative industry-academia projects stretch its chemistry into new territory, seeking even greater reactivity control without losing resistance. Scientists in polymer engineering believe longer life, lighter weight, and unmatched durability all start with intermediates like this one.
Toxicity Research
Regulatory agencies watch new fluorinated aromatics closely because past cousins in the PFAS family created environmental headaches. Early reports on 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol put its acute toxicity well below common industrial solvents. Toxicological profiles show low bioaccumulation and minimal oral or inhalation toxicity in small animals under controlled doses. Yet, manufacturers remain cautious since chronic exposure or breakdown byproducts might lead to issues science hasn’t mapped out yet. Labs push to understand its fate in soil and water ecosystems. The push for “greener” fluorinated intermediates springs from lessons learned: today’s innovation must respect future environmental limits. Waste disposal standards reflect these concerns, nudging users away from careless handling.
Future Prospects
Across the next decade, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol sits at the threshold of bigger commercial application, especially as electronics and industrial coatings become more demanding. Every time industry asks for lighter, tougher, and more resilient plastics, chemists hunt for platforms with this sort of robustness. Environmental scrutiny will only intensify, so scalable green synthesis and full lifecycle assessment gain attention in research portfolios. Expect chemistry journals to spotlight new ways to recycle or upcycle residual fluorinated intermediates into benign products. If handled wisely, this molecule can accelerate progress in electronics, transportation, and protective coatings, all without repeating mistakes from the last wave of fluoropolymer growth.
The Everyday Reality of Specialty Chemicals
In the world of specialty chemicals, some names look intimidating but play a quiet role in things people interact with daily. One of those is 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol. Not something you see on the shelf at the local store, but its presence touches quite a few manufactured products that matter both inside industries and outside, with every person who uses an electronic device.
A Building Block for Modern Polymers
This compound shows up most often in places that demand materials to perform well under tough circumstances. It’s mainly used in the world of polyesters and polycarbonates. These classes of plastics form the backbone of electronics and medical equipment. The added fluorine atoms give finished materials powerful resistance against heat, harsh cleaning chemicals, and water. Instead of yellowing or breaking down, they keep their shape and their performance for years. I’ve seen old optical parts that still look nearly new thanks to the smart choices chemists made with monomers like this one.
Electronics Rely on Smart Chemistry
Circuit boards, connectors, and insulation for wires—many of these grab advantages from specialty plastics made using this tetrafluorinated compound. The fluorine content knocks down unwanted static electricity, which really matters in high-tech items where a single spark can mean disaster. Even in tiny quantities, it gives polymers a boost in stability during rapid heating and cooling cycles. Many people have never realized how much the safety and reliability of their gadgets depend on behind-the-scenes chemistry.
Better Performance in Medical and Optical Devices
Once you step into medical technology, the same properties that help electronics also show value in items like surgical tools, housings, and lighting equipment. The industry faces tough standards because patient safety is on the line. Polymers manufactured with 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol show clarity that rivals glass, yet they don’t shatter. They also let engineers design lighter tools, which makes everybody’s work easier. It’s not just about toughness—hospitals run harsh sterilization cycles, and the plastics need to hold up to constant treatment without leaching chemicals or failing over time. Mistakes aren’t allowed. Companies building these devices go for reliable raw materials to reduce risk, and fluorinated compounds deliver.
Weighing Safety and Environmental Concerns
Like many things in modern chemistry, balance matters. Fluorinated materials raise concerns about persistence in the environment and health safety, especially if they ever break down or escape during manufacturing. The industry knows this. Tight regulations track industrial emissions and handling procedures. Over the years, the move toward greener chemistry put momentum behind research on alternatives and recycling of fluorinated polymers to avoid dumping them in landfills or water supplies. Transparency and rigorous oversight keep risks down. Responsible sourcing and safe disposal practices must stay front and center as demand for advanced materials grows.
Supporting Future Innovation
Specialty compounds like 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol aren’t flashy, but they power the next wave of durable, high-performing materials. As electronics get smaller, and medical devices get even more demanding, these chemical building blocks pull invisible weight behind the scenes. The challenge lies in pushing for performance while always keeping an eye on environmental impact and health. That comes down to careful scientific work, strong oversight, and a willingness to invest in safer, smarter ways to keep these innovations moving forward.
Understanding the Molecule at Its Core
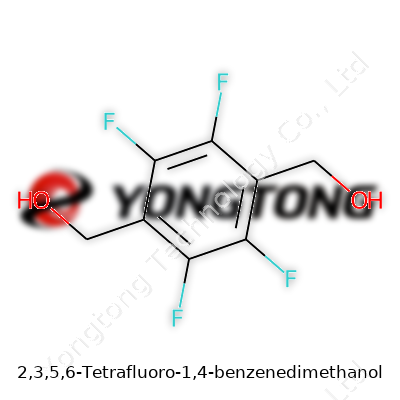
2,3,5,6-Tetrafluoro-1,4-benzenedimethanol packs a punch in the world of specialty chemicals. Its molecular formula stands as C8H6F4O2. The molecule starts with a standard benzene ring, but in this case, four hydrogen atoms get swapped out for fluorine on the 2, 3, 5, and 6 positions. The ring then picks up two hydroxymethyl groups at the 1 and 4 spots. Plenty of chemists know this substitution pattern makes a real difference, drastically changing how the molecule behaves compared to its plain hydrogen cousin.
Mapping the Structure
Drawing the structure in your mind starts with a benzene ring — six carbon atoms holding hands with alternating double bonds. Mark positions 2, 3, 5, and 6 with fluorine atoms, highly electronegative elements that pull electron density their way, and you get a non-polar region chopped up by powerful polar bonds. At the 1 and 4 positions, the ring sports -CH2OH groups, not just tacking on more hydrogen and oxygen but creating points for potential reactivity. For those needing a SMILES code, it looks like: OCc1c(F)c(F)c(CO)c(F)c1F.
Chemical Importance
As someone who’s handled all sorts of specialty monomers and intermediates, this chemical sticks out for its balance between strong electron-withdrawing fluorines and those friendly, reactive alcohol groups. The fluorines turn the aromatic core into a fortress, resisting degradation and offering up new properties, like lower polarizability and increased chemical stability. The dimethanol groups offer the synthetic flexibility needed to tether polymer chains, cross-link structures, or build out into more complex molecules. Materials science leans on molecules like this for performance plastics and resins, thanks to that unique combo of stability and reactivity.
Real-World Uses and Potential
Polymer chemists have long used compounds with multiple hydroxymethyl groups to create robust, high-performance resins, especially when those alcohol functionalities slot in for further reactions such as esterification or etherification. The addition of fluorines onto the aromatic ring goes a step further, offering properties like increased fire resistance, lower dielectric constants, and better resistance to solvents. Electronics, aerospace, and coatings industries look for such attributes. Knowing how versatile the molecule is gives formulators room to play, tweaking final material properties by swapping out similar aromatics with or without fluorines.
Some Challenges Don’t Stay Hidden
Getting your hands on fluorinated building blocks doesn’t come cheap. Fluorine atoms can seriously complicate both synthesis and waste disposal. Experience tells me that working with polyfluorinated intermediates requires solid training, good gloves, and careful waste management protocols. The same chemical toughness that helps in end-use translates to hurdles in breaking these materials down, whether in the lab or after product life ends. Environmental chemists highlight the need to watch out for persistent organic pollutants. Balancing the demand for advanced material performance with environmental responsibility puts pressure on producers to innovate cleaner, safer, and more sustainable methods for making and recycling these compounds.
Room for Improvement
For researchers, finding catalytic techniques that lower production costs and reduce the use of hazardous reagents can turn groundbreaking chemicals into mainstream products. Exploring bio-based routes or milder reagents can play a part in making molecules like 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol available without harsh trade-offs. People working at the bench and on the production floor see the value in every tweak that keeps workers safer and prices more stable.
Looking Forward
Working with complex aromatic alcohols, especially those decked out with multiple fluorines, continues to push material science forward. By blending bench experience with ongoing research, heavy-hitting specialty chemicals like this one can stay both effective and responsible in tomorrow’s market.
Why Correct Storage Really Matters
Roots of good chemical stewardship grow from paying close attention to detail. I’ve noticed plenty of smart people skip over storage steps thinking nothing could go wrong, but a small misstep with something like 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol changes things quickly. This compound brings both potential as an intermediate in synthetic chemistry and real risk if handled too casually.
Understanding Sensitivities
Anyone with lab experience picks up on the quirks of each material. This compound doesn’t take kindly to moisture. Even letting the cap sit loose during weighing lets in the humidity, pushing the purity downhill and changing reaction outcomes. It doesn’t do any favors for lab budgets or for safety.
Fires or exposure disasters rarely start with dramatic events; a few small drips or failing seals matter more. Poly-fluorinated compounds often bring long lifespans in the environment. If a bottle leaks in storage, the trouble doesn’t wipe away with a towel. Neighbors in the chemical cabinet sometimes cross-contaminate as well, which means segregating this compound from acids, bases, and oxidizers isn’t just a checklist step—it saves headaches in the long run.
Choosing the Right Container
Some solvents chew through thin plastics, but this substance holds up well in amber glass containers with PTFE-lined lids. Glass resists any reactions and holds a tight seal. That PTFE liner earns its keep—regular caps fail too quickly, leading to slow leaks. Fooling around with plastics, unless specified, can risk absorption or reaction.
Opaque or amber glass blocks out excess light. Sunlight decomposes white powders and colorless solids faster than most expect, so working in a windowless space or using closed cabinets makes a difference.
Temperature Range and Humidity
Dry, cool rooms make the best home for organic reagents like this. Many labs set the thermostat between 15°C and 25°C. Higher temperatures nudge up degradation or even vapor pressure, which means more fumes. Damp basements or high-humidity, unventilated corners go on the “avoid” list.
Desiccators work for small amounts—simple silica gel packets block out enough moisture to make a real improvement. If storing larger quantities, keeping the bulk container sealed, then aliquoting for bench work, keeps exposure time short.
Access and Labeling
One weakness in every lab: mystery containers, no date, no initials. Every bottle of 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol deserves a clear label with the full name, date received, and hazard symbols. Proper organization keeps new students or visiting researchers from grabbing the wrong thing in a pinch.
Emergency Considerations
I once saw a bottle of an organic solid split open after a careless stacking job. Secondary containment—a simple bin or tray—catches leaks before they reach the floor. Spill kits and fresh gloves nearby mean reactions stay contained, and nobody gets exposed by running across a sticky countertop.
Good housekeeping beats fancy storage technology every time. Regularly checking seals, watching for crystals around caps, and rotating stock go further than the best emergency guide.
Daily Practice in the Real World
In the end, mindfully storing 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol doesn’t just protect current projects; it builds a safer and smarter workplace. Skipping corners might save minutes but ends up costing more. I’ve learned the hard way: detailed care on the shelf pays off in discoveries at the bench.
Why Safety Matters in the Lab
Anyone who’s spent time working with chemicals knows that every new substance comes with its own quirks. In my experience, a consistent rule stands out: Don’t assume a low-profile chemical can’t surprise you. 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol may not leap out as an obvious hazard, but behind those tightly sealed reagent bottles, it joins a long list of compounds that deserve real respect.
The Real Risks
This specific fluorinated benzenedimethanol packs a punch in toxicity potential. Its molecular structure sets up unique dangers—fluorinated aromatic compounds tend to be sneaky, and those -OH groups can lead to skin or eye irritation. Spills dry fast, but leave behind residues that keep giving. One careless splash, even from a small pipette, can lead to a surprise rash. Most research shows that respiratory irritation isn’t uncommon with dust, so popping the cap in an unventilated prep room turns routine weighing into an unnecessary risk.
Beyond direct contact, I think about long-haul exposure too. While there isn’t a lengthy record for this specific compound, similar structures have a track record of making gloves and regular goggles seem like afterthoughts. Solvents mixed with tetrafluoro aromatics can transport the compound through common nitrile gloves before a busy hand even notices. I’ve watched newcomers assume thicker gloves give immunity, only to regret it after a long afternoon handling reactive powders.
Precaution Starts Before the Bottle Opens
No substitute exists for real planning. Start with a clean workspace—clutter leads to accidents. Before weighing or transferring, double-check gear: butyl rubber gloves trump thin nitrile, and a sturdy lab coat with full sleeves matters more than the in-laundry hoodie. Chemical splash goggles often fit clumsily, but fit counts more than comfort when powders or splashes threaten.
Fume hoods save skin and lungs. The temptation to weigh out ‘just a little’ on an open bench wastes odds in your favor. I’ve seen even careful users lean away from a balance, thinking distance alone will help. One gust of air pushes the dust around. For any solvent manipulation or solution mixing, ventilation isn’t a bonus—it’s protection.
Spills and Storage
Spills happen. I always keep a spill kit close: absorbent pads, neutralizing agents if possible, and a full face shield. It’s easy to think of quick wipes as enough until a reaction starts with the cleaning paper itself. Handle spills slow and steady, especially with fluorinated compounds that react with many organics and aren’t always friendly on surfaces.
Storage stands between today’s experiments and tomorrow’s accident. Store away from heat and acids. These compounds often react unexpectedly if left near incompatible reagents. Clear labels and a tidy, well-ventilated space give you a second chance if things get knocked over or mixed up by accident.
Building a Culture of Caution
After years hands-on in the lab, real safety doesn’t begin with fancy posters. It comes from understanding the chemical you’re working with and from learning how to take each step deliberately. Training new lab members to ask questions and respect risks keeps everyone on the right track. Don’t rush, don’t guess, and don’t cut corners—especially not with fluorinated alcohols like 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol. Every small choice adds up to a safer lab and a longer, healthier career.
Sourcing Specialty Chemicals: Sometimes It’s a Maze
Ask anybody in chemical research or specialty manufacturing how many hours get eaten up just tracking down rare reagents, and you’ll hear stories. One of those tongue-twister molecules, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol, can turn even the simplest shopping list into a scavenger hunt. Most folks look for it through established lab suppliers—the kind of shops universities and pharmaceutical companies trust. Sigma-Aldrich, ChemShuttle, Oakwood, Toronto Research Chemicals, and abcr GmbH have this one in their online catalogs, though it skips in and out of stock depending on the season and demand.
Supplier Choice and Trust
Choosing a supplier always involves tradeoffs. Larger distributors offer quality control, a solid trail of paperwork, and some confidence that nothing got mixed up. Shady or little-known vendors might dangle what looks like a cheaper price, but the risks soar—impurities, unreliable shipping, missing documentation. Chemical supply remains one of those rare industries where trust and paperwork run parallel with science. Much of that traces back to regulatory requirements. Safe handling of fluorinated compounds typically demands professional oversight, and suppliers don’t ship to just anyone. Expect to provide a business account, credentials, and a use case if you request a quote.
What the Price Tag Says About This Compound
The going rate surprises people at first glance. Drilled down, 2,3,5,6-Tetrafluoro-1,4-benzenedimethanol sits in a class of molecules where manufacturing complexity, purity demand, and small batch production keep the price above basic solvents and basic reagents. Over the past year, quotes from major suppliers mostly sit in the $250 to $450 range for a gram, and prices tend to drop slightly if you buy larger batches. This is nowhere near the level of peptide reagents or custom oligonucleotides, but it isn’t pocket change either. Handling cost also piles up—extra packaging, hazardous labeling, air-locked shipping. As with many fluorinated organic chemicals, price dances on global supply chain issues, access to precursors, and regulatory environments.
Why Price Remains so Stubbornly High
Fluorinated benzene derivatives don’t appear on every synthetic chemist’s bench. Their manufacture requires multiple production steps, exacting purification, and strict lab safety. Fluorinated intermediates pose inhalation and handling risk, which drives up insurance premiums at every step. These costs don’t just live on the books; they show up right in the invoice. Demand remains niche, so economies of scale rarely kick in, unlike with commodity chemicals.
Ways to Ease the Burden for Small Buyers
There’s no secret shortcut, but researchers can tap academic purchasing programs or industry consortiums to share orders. At some universities, colleagues round up their lists every couple of months and pool resources. Some suppliers offer “cut-and-aliquot” programs, splitting manufacturer lots among university or corporate clients. It doesn’t make the compound cheap, but it spreads the pain. Vendors also allow for custom synthesis, though minimum order quantity often wipes out savings unless the order grows to several dozen grams or more.
Regulations Shape the Marketplace
Purchasing rules exist for strong reasons. Tighter control stops diversion into irresponsible hands and keeps hazardous chemicals traceable. In some countries, import and export laws link directly to environmental and health risk assessments, which tacks extra costs onto cross-border orders. Prospective buyers save hassle by getting clear on local regulations before placing an order—something that never appeared in textbooks, but proves essential in practice.
Looking Forward
As technology pushes demand for custom fluorinated intermediates in electronics, batteries, and pharmaceuticals, suppliers may be convinced to adjust pricing, but right now, nobody sees a new normal arriving soon. In a world where every reagent feels critical to a discovery, these costs shape research as much as infrastructure and labor.