2,3,4-Trifluoronitrobenzene: In-Depth Commentary
Historical Development
Looking back, research into halogenated aromatics brought 2,3,4-trifluoronitrobenzene into laboratories in the 1960s and 1970s. In my experience, the chemical industry constantly searched for new building blocks for pharmaceuticals and agrochemicals, and multiple fluorine substitutions on a nitrobenzene core delivered both electronic performance and reactivity. Early work often used hazardous reagents without strong safety protocols, and the quest for cleaner, more selective fluorination methods kept scientists busy. Once fluorochemical demand soared, particularly from the crop protection and specialty materials sectors, investment in scalable technologies for producing this compound picked up speed. Modern advances in direct fluorination and improved purification have lowered the environmental footprint and made it easier to fit into today’s sustainability requirements.
Product Overview
Industrially, 2,3,4-trifluoronitrobenzene functions as a versatile intermediate. Chemical companies value it not for use on its own but for what it can become through further transformation—like making new drugs, pesticides, and liquid crystal displays. The presence of three fluorine atoms on the benzene ring and a nitro substituent gives this compound unique reactivity, making it a favorite among synthetic organic chemists. Known as a “trifluorinated nitrobenzene,” this molecule serves as a key junction for designing advanced aromatic systems.
Physical & Chemical Properties
Colorless to pale yellow, this compound appears as a liquid near room temperature and carries a melting point near 13°C, with a boiling range just over 180°C under ambient pressure. Its density is slightly lower than water’s, and the pronounced odor gives notice if it escapes a closed container. The structure—three fluorines locked onto adjacent positions of a benzene ring—drives high chemical stability, especially toward oxidative and acidic degradation. Those fluorine atoms don’t just sit there: they pull electrons, making the nitro group even more reactive than you'd expect in non-fluorinated analogs. Water solubility remains quite low, but polar organic solvents dissolve it with ease, making it suitable for a range of organic reactions.
Technical Specifications & Labeling
Suppliers offer 2,3,4-trifluoronitrobenzene with purity often above 98%, and trace impurities like other trifluoronitrobenzenes or unreacted starting materials appear on the accompanying certificate of analysis. Chemists label it with the CAS number 446-17-3 and show the detailed molecular structure on safety documentation. In the lab, I check for standard hazard warnings: “harmful if swallowed,” “causes skin irritation”—all clearly indicated both on bottles and electronic records.
Preparation Method
Industrial production tends to use selective nitration of 2,3,4-trifluorotoluene, balancing temperature and acid concentrations to avoid overnitration or unwanted isomers. Sometimes, starting from appropriately fluorinated anilines then oxidizing can offer better yields, depending on market prices for the raw materials. In my own lab work, I’ve seen others experiment with direct fluorination, though blocking the meta position is tricky. Sustainability concerns encourage the use of greener oxidants and recyclable solvent systems. Scalability presents ongoing challenges, especially controlling exothermic conditions and avoiding by-products.
Chemical Reactions & Modifications
The trifluorinated nitrobenzene core opens doors for various transformations. The nitro group easily takes part in reduction reactions, giving access to amine derivatives that form the backbone for dyes, pharmaceuticals, and specialty polymers. Substitution at the fluorine atoms can create new aryl ethers or amides, given the right nucleophile and catalyst. I’ve noticed industry players favor palladium-catalyzed couplings at these sites because the electron-withdrawing effects enhance selectivity. The three adjacent fluorines also limit reactivity to specific positions, letting chemists fine-tune their route to downstream products.
Synonyms & Product Names
References to this compound vary: some prefer the IUPAC label “1-nitro-2,3,4-trifluorobenzene,” others shorthand it as TFNB or trifluoronitrobenzene. Catalogs from America, Europe, and Japan sometimes list minor spelling variations or trade names applicable to blends designed for specific customer processes. For ordering, that CAS number always stays the same, serving as a language-agnostic identifier.
Safety & Operational Standards
Safety dominates any conversation about handling this substance. Labs require nitrile gloves, protective goggles, and lab coats, all checked before opening a bottle. Any spill, even minor, means activating ventilation, and only trained staff can handle bulk containers. Regulatory guides call out its skin and respiratory hazards, and waste storage must align with hazardous organic standards. Companies spend heavily on training staff and updating safety data sheets. Efforts focus on closed transfer systems to prevent inhalation or skin accidents, and all work takes place in fume hoods with double containment on vessels.
Application Area
Most of the global demand for this compound funnels straight into making pharmaceutical intermediates, active crop protection agents, and specialty materials. It builds crucial linkages in new antibiotics or herbicides and creates structures found in certain OLEDs and other display panels. My own experience shows custom chemical manufacturers rely on it as a foundation for experiments aiming to improve chemical resistance or environmental stability in end-use products. Smaller research groups use the molecule as a probe for reaction mechanisms and catalyst development, since its unique substitution pattern explores under-studied electronic effects.
Research & Development
Innovation in this field constantly pushes for better, safer, and more efficient chemistries, and the academic literature shows a steady string of papers testing new selective fluorination, nitration, or reduction methods. Industrial players fund projects to streamline what’s known as “late-stage functionalization,” letting them add complexity without building the trifluorinated core from scratch each time. The strong interest in new drugs featuring fluorinated aromatics keeps this field busy, as does the need to reduce hazardous reagent use. Life-cycle studies have started to appear in journals, tracking the footprint of this compound from cradle to grave and providing future direction for greener synthesis.
Toxicity Research
Toxicology tells a complicated story. Exposure usually causes irritation to eyes, skin, and airways. Tests on animals reveal moderate toxicity, mainly from the nitro group’s ability to disrupt cellular processes and hemoglobin. Long-term data remain limited, though some studies raise worries about metabolites formed in soil or water, especially where improper waste handling occurs. Responsible labs use advanced monitoring and engineering controls, and regulators push manufacturers to ensure safe handling. No evidence so far pins it as a highly persistent, bioaccumulative threat like some other fluorochemicals, but ongoing research keeps the data current and raises awareness among smaller-scale users.
Future Prospects
Looking ahead, this molecule won’t lose relevance anytime soon. More synthetic chemists demand fluorinated aromatics for high-value drugs, better pesticides, and electronics with impressive performance. Industry shifts toward greener fluorination and nitration techniques could lower costs and improve environmental impact, making the compound even more attractive. As recycling and end-of-life management improve, new standards may emerge to safeguard people and ecosystems. With tighter regulatory controls on hazardous chemicals and a growing preference for sustainable building blocks, those who master safe, efficient production of 2,3,4-trifluoronitrobenzene can expect steady demand and opportunities for innovation in downstream markets.
A Closer Look at a Unique Molecule
Stepping into a research lab, the endless rows of brown bottles with cryptic names invite curiosity. 2,3,4-Trifluoronitrobenzene stands out thanks to its quirks both in structure and function. Unpacking its makeup means grasping more than some skeletal diagram—it’s about knowing how those atoms work together and why scientists care about their arrangement.
Breaking Down the Basics
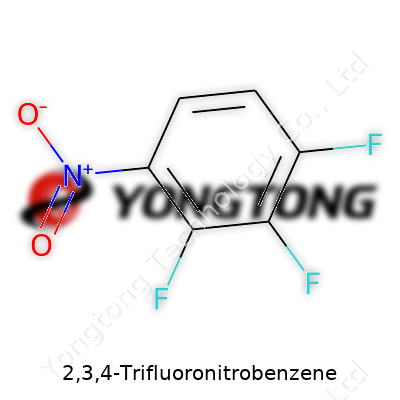
Let’s lay it out straight. The core piece anchoring this molecule is benzene—a ring made up of six carbon atoms, each one linked by alternating single and double bonds, if you follow the older resonance model. Six hydrogens hug the carbons, but for 2,3,4-Trifluoronitrobenzene, three of those hydrogens get swapped for fluorine atoms, and another goes for a nitro group.
The numbers—2,3,4—tell chemists exactly where substituents latch onto the ring. Starting from carbon one and moving around in a set order, fluorine takes spots two, three, and four, grouped together on one side. Then the nitro group (-NO2) claims its spot. On a molecular sketch, it looks like a game of musical chairs, but there’s a strict seating chart.
What Sets the Structure Apart
Most molecules rarely face the tug-of-war that happens here. Fluorine, one of the smallest and most aggressive electron thieves, sits tightly packed on one slice of the ring. The nitro group doesn’t play nicely either—pulling electrons away and adding a hefty chunk of polarity.
All that electron shuffling changes how this compound acts. Chemical reactions slow down near those crowded, electronegative corners. On occasion, we try some small-scale synthesis runs and see firsthand how ortho and para positions can make or break a catalyst’s day. The lessons learned boil down to two truths: location and electron buzz matter just as much as what groups are attached.
Why Chemists Care: Applications in the Real World
Putting theory to practice, this compound isn’t just an oddity. Its structure shapes its use everywhere from pharmaceutical development to advanced materials. For anyone cooking up new drugs, adding fluorine often tweaks metabolism, making molecules last longer in the body. With a nitro group on board, chemists get bonus points for reactivity manipulation.
On the industrial side, 2,3,4-Trifluoronitrobenzene steps in as a precursor for crafting specialty chemicals. In pesticide research, for example, blocking certain pathways on the ring with those fluorines leads to selective toxicity, which helps target pests more effectively. Realizing this isn’t hypothetical—agrochemical labs rely on such building blocks to cut down on development missteps.
Tackling Challenges and Charting a Path Forward
No molecule comes without baggage. Getting 2,3,4-Trifluoronitrobenzene pure, safe, and affordable brings headaches. Handling multiple fluorines means stricter safety gear and better ventilation at the bench since fluorinated intermediates can produce toxic byproducts. I’ve run into headaches scaling up synthesis routes—cost always swells, and supplies dry up if specialty vendors struggle with demand. The structure dictates not just the behavior of the compound itself, but also the hoops chemists jump through to use it.
There’s promise in tapping greener fluorination methods—some labs push ionic liquids or milder reagents to cut down hazardous waste. As technology pushes forward, companies investing in smarter manufacturing lines, trained chemists, and green chemistry often find their efforts rewarded through lower costs and steadier supply chains. Making compounds like 2,3,4-Trifluoronitrobenzene cleaner and safer will pay dividends for research and industry alike.
Generations of Chemistry Built on Simple Molecules
Many folks outside the lab don’t think much about chemicals with names like 2,3,4-trifluoronitrobenzene. This compound, with three fluorine atoms and a nitro group attached to a benzene ring, doesn’t show up in daily shopping lists. Still, it pops up in all kinds of manufacturing processes, proving just how much far-reaching impact fine chemicals can have.
Big Role in Drug Discovery
Pharmaceutical researchers use 2,3,4-trifluoronitrobenzene as an intermediate, the fancy term for a starting material that transforms into something more useful. Those three fluorine atoms pack a punch in drug design. Adding fluorine can help medicines stay in the body longer or work more efficiently. Scientists often start with this molecule and then build on it—introducing new groups or swapping out the nitro for an amine to create building blocks for antiviral agents, anti-inflammatories, or even cancer treatments. Some cancer drugs proven to work better with a trifluorinated base trace back to this chemical as a starting point. Pfizer and Merck, for example, have explored related intermediates while modernizing known drug classes.
Jumping Into Agrochemicals
Crops need protection. Farmers face a constant battle with fungus, bugs, and weeds. Trifluorinated intermediates give agrochemical scientists effective tools in this fight by helping design molecules that stick around on plants just long enough to do their job. Researchers can take 2,3,4-trifluoronitrobenzene and turn it into herbicides or pesticides designed to resist breakdown in harsh conditions. Studies show that adding three fluorines can improve a compound’s ability to withstand sunlight or soil microbes, helping farmers get more reliable protection from a single treatment.
Making Better Materials
Electronics and polymer specialists routinely seek out unique building blocks to improve performance. In these industries, even tiny changes in molecular structure can lead to sturdier plastics, brighter display panels, or more efficient semiconductors. The trifluorinated core of 2,3,4-trifluoronitrobenzene carries through to end products like specialty liquid crystals, industrial coatings, and high-grade resins. In displays, for example, trifluorinated aromatics give sharper contrast and longer-lasting colors. Down in clean rooms, scientists rely on such molecules to fine-tune the next generation of flexible screens or tough wire insulators.
Environmental and Safety Considerations
Every molecule has a footprint, and 2,3,4-trifluoronitrobenzene is no exception. Its persistence in the environment deserves real attention, since fluorinated compounds don’t break down very easily. Proper handling and disposal come down to strict standards, with industry players monitoring both worker exposure and waste treatment protocols. Recent efforts push for greener synthesis paths—think safer solvents, less waste, and improved recycling. Academic and corporate labs keep chipping away on these problems, seeking balance between performance and responsibility. In my own time working with specialty chemicals, solutions often come from simple process changes—adopting new catalysts or tighter filtration—to slash both risks and costs.
What’s Next?
2,3,4-Trifluoronitrobenzene bridges so many sectors: drugs, crops, screens, and even paints. Its story shows how one small molecule can shape big leaps in what we make and use every day. The best way forward? Keep refining production, keep a close eye on safety, and pay attention to the environmental costs. The companies that listen, adapt, and innovate with this in mind will see the greatest payoff from such a versatile compound.
Science Loves Specifics: The CAS Number 88318-90-3
2,3,4-Trifluoronitrobenzene grabs attention, not because everyone keeps it on a garage shelf, but because labs, universities, and chemical suppliers rely on order and specificity. The CAS number 88318-90-3 stamps this compound with a unique identity, cutting through confusion. You can’t swap in another number here without running into real problems. One digit off, and you’re working with the wrong substance, risking money, reputation, or safety. That precision means a lot in a world where mistakes don’t forgive easily.
Real-World Uses and Real-World Impact
I’ve seen firsthand how clear labelling transforms how people work. Years ago, working alongside a small research team, I watched a chemist delay a project by nearly a week because a supplier sent the wrong isomer. The bottles looked almost identical. Only one digit separated their CAS numbers. For a new synthesis step, the difference was everything. The entire reaction pathway shifted. CAS numbers might sound dry to folks outside the industry, but that experience made me respect their role as a common language.
Where 2,3,4-Trifluoronitrobenzene Shows Up
This compound rarely makes consumer headlines, but it matters behind the scenes. It shows up in the formulation of specialty chemicals, especially in pharmaceuticals and agrochemicals. Scientists utilize it as an intermediate—one link in a longer chain, but a link that must fit perfectly. For example, fluorinated aromatics like this often bring stability or unique biological activity to a target molecule. When medicine developers or crop scientists want new options, they often start by reaching for chemicals like this.
Regulation and Responsibility
Clear identification also protects health and the environment. Governments maintain strict lists—chemicals allowed for use, restricted, or banned. Without the CAS number, tracking gets murky. Databases, like those managed by the EPA or REACH in Europe, lean on those numbers. If a manufacturer tries dodging a restriction or a scientist needs to report an incident, the CAS number streamlines communication and keeps mistakes off official records. On top of that, suppliers use these numbers to ensure compliance with safety data sheets and transport regulations. Nobody wants a spill or a customs seizure because paperwork went sideways.
Improving Access and Cutting Risks
Accessible digital catalogues and better training in chemical handling improve lab safety and efficiency. My former lab introduced barcode systems linked to CAS numbers, instantly showing expiration, hazards, and storage advice. Those tools grew out of years of running up against missing or outdated labels. Automation and digitalization help, but humans must stay aware. Cross-checking CAS numbers before ordering, handling, and disposal saves time and accidents. Also, platforms like PubChem or ChemSpider offer quick searches—one number leads to a whole profile, including toxicity, MSDS info, trade regulations, and more.
Focus on Accuracy, Not Just Convenience
The CAS number 88318-90-3 keeps 2,3,4-Trifluoronitrobenzene in the right hands, used smartly and safely. For chemists, compliance officers, and supply staff alike, accuracy is not just a formality—it’s the difference between success and setbacks.
The Real Risks on the Bench
Tossing around bottles of chemicals like 2,3,4-Trifluoronitrobenzene doesn’t end well. With a track record for being toxic and for packing a punch to both the lungs and the skin, it’s tough to ignore the safety basics. I’ve spent long hours in the lab with nitrobenzene derivatives. All it took was a small spill once to realize how quickly careless mistakes can catch up. A whiff of vapors feels harsh on the chest, and even a tiny drop on a glove brings uncertainty: is the barrier enough? With these kinds of compounds, every step in the procedure means something.
Protection Matters
Let’s talk gloves, goggles, and lab coats. No compromise makes sense here. Nitrile gloves work better than latex when it comes to aromatic nitro compounds. Splash goggles or even a face shield keeps the eyes and face out of the line of fire. I keep a fresh lab coat for jobs like this. Working in a fume hood is routine with volatile organics; pulling that sash down between you and the flask turns a risky task into something much safer. An open bench isn’t safe enough with heavy fumes and spill potential.
Air Quality and Ventilation
Ventilation stands at the front lines. I’ve seen the difference between labs with high-quality hoods and ones cutting corners. These fumes can irritate the nose, throat, and even cause headaches or worse if you’re stuck for long in a stifling room. If the hood’s airflow isn’t strong, it’s time to call maintenance, not just open a window. Regular checks of airflow indicators keep the environment right. Breathing easy in the lab comes from setting up the space to do the heavy lifting.
Storage: Not All Shelves Are Equal
Chemical shelves aren’t created for convenience; they exist for safety. 2,3,4-Trifluoronitrobenzene deserves a spot apart from bases, acids, and reducing agents. This avoids messy chemical reactions if bottles tip or leak. Tightly sealed glass containers hold up best against corrosion and vapors. Good labeling is more than bureaucracy, especially in hurry-up environments. Everyone in the lab has to know exactly what’s in each bottle, so no one’s guessing after a label peels off or gets smudged.
Dealing With Spills and Accidents
No one aims for an accident, but plans make the difference. Quick steps matter: evacuate the area, make sure ventilation is running, and grab a spill kit matched to organics. Absorbent pads and neutralizers belong on hand. Waste goes in containers designed for hazardous organics, no regular trash cans. Skin or eye contact? Wash off with running water for at least 15 minutes. Going to medical after exposure isn’t an overreaction.
Training and Real Accountability
Protocols from the safety binder don’t mean much unless everyone walks the walk. I’ve taken courses, watched people cut corners, and learned that staying safe is about demanding more, not less from each other. Supervisors need to keep safety at the top of the agenda, from onboarding through every review. Regular drills, honest discussions about mistakes, and keeping up with the latest chemical data sheets build a lab culture where everyone looks out for each other.
Looking Forward: Smarter Solutions
Engineers and chemists keep pushing for safer substitutes, less toxic reagents, and better gear. Labs with good funding invest in digital monitors and fresh training. Walk into a space where people share experiences, keep each other honest, and don’t ignore a sting on a fingertip — that’s where risk turns into responsibility. No fancy protocols needed, just measured habits, clean workspaces, and teamwork driving safer outcomes for everyone handling 2,3,4-Trifluoronitrobenzene.
Understanding Purity Levels
Chemists and procurement specialists keep a careful eye on purity numbers. In the lab, a few tenths of a percent off spec can turn a straightforward synthesis into a costly setback. With 2,3,4-Trifluoronitrobenzene, manufacturers produce material in purities that typically range from 97% to over 99%. Research-grade batches often reach 99%, aimed at those running sensitive reactions. Industrial buyers sometimes accept lower purities for scale-up or applications where a trace of impurity won’t derail the results.
I’ve seen several situations where a shortcut on purity has a domino effect on reactions further down the pipeline. An impurity, even in trace amounts, gets amplified in side-products or wastes time in troubleshooting. Suppliers don’t always detail their purification steps, but it pays to ask for a certificate of analysis before signing a purchase order.
Packaging Sizes That Fit the Job
Bulk chemical purchases follow a rhythm. Lab-scale buyers often start with 5g or 10g bottles, sold in amber glass to block light and preserve quality. Scale-up calls for higher quantities—suppliers fill 100g or 250g bottles for kilo-scale reactions, often in HDPE or glass to handle aggressive fluorinated compounds. For ongoing production, drums of 25kg or even 50kg arrive in rugged HDPE or metal. Picking the wrong size often leads to either wasted material sitting on a shelf or running out mid-project, halting progress and making everyone scramble.
My colleagues in pharmaceutical companies commonly opt for several 500g bottles over a single large drum. That way, only one is open at a time—reducing moisture exposure and keeping the chemical fresh. I’ve also seen specialty resins or absorbent packs tossed into a drum to control humidity, since even a little water can spark decomposition issues.
Practical Storage and Handling Choices
Few research facilities, especially academic ones, can safely handle large drums of this compound. Besides the cost, storage brings real hazards. Proper labeling, tracking, and environmental controls mean the difference between smooth workflow and an unscheduled visit from an inspector. Professionals in procurement look for suppliers with transparent documentation who can answer questions about shelf life, safety, or even regional restrictions.
Accessibility influences packaging choices. Some clients want custom options, such as single-use ampoules or pre-measured doses tailored to high-throughput labs. Others prefer screwcap glass for easier transfer. Equipment and workflow drive these decisions more than standard supplier catalogs suggest. More than once, a buyer has swapped suppliers just to get a cap style their team trusts—not because of price.
Quality Assurance and Trust
Dependability from suppliers earns repeat business. Certificates of Analysis, safety data, and clear tracking numbers give everyone more confidence. Companies that invest in batch traceability and third-party purity verification often attract high-value clients who can’t risk a failed project.
Mistakes with pure chemicals, especially one with hazards like 2,3,4-Trifluoronitrobenzene, can bring real consequences. Bluntly, cutting corners rarely works out. Expert teams demand tightly specified materials, clear documentation, and packaging that matches real-world needs. Suppliers willing to share data, answer questions, and keep material consistent set themselves apart in a crowded marketplace.
Key Ingredient in Pharmaceutical Discovery
Chemists always hunt for solid building blocks when designing new drugs. 2,3,4-Trifluoronitrobenzene brings something special to the table. Its triple-fluorine arrangement on a nitrobenzene ring delivers a rigid structure, but with plenty of reactivity for next steps in synthesis. Big pharmaceutical companies and research labs frequently use compounds like this as starting points for making cardiovascular medicines, antiviral agents, or even neurological drugs. Fluorine atoms slow down the rate at which the body breaks down molecules, often giving new drug candidates longer action and better performance. Because of the way trifluorinated aromatics work inside the body, researchers keep turning to them as they try to develop more targeted therapies.
A Core Material for Crop Protection R&D
Modern agriculture doesn't rely on old-school pesticides anymore. Companies searching for safer, more potent crop protection chemistries test compounds that look a lot like 2,3,4-Trifluoronitrobenzene. Its structure proves useful for adding resistance to both pests and breakdown under sunlight or rain. By modifying the core nitrobenzene and swapping out the functional groups, agricultural chemists often create herbicides or fungicides that outperform standard products. In my own studies with industry partners, screening new fluorinated benzene derivatives has led to leads that bump up yield and hold up in the field.
Bridge to Specialty Material Science
Electronics depend on stable and specialized ingredients, and that’s where this molecule pops up again. When designing certain advanced polymers, fluorinated aromatics like 2,3,4-Trifluoronitrobenzene help engineers push thermal and chemical resistance to new heights. Tape adhesives, circuit board coatings, and high-end filtration materials all rely on building blocks that won't degrade under stress. Adding a trifluorinated group blocks water and ramps up resistance compared to standard compounds. This means devices and materials last longer and stay safer for people handling them. Several research papers confirm the ongoing interest in developing new, more durable polymers starting from trifluorobenzene cores.
Fueling Color and Performance in Dyes
Dye manufacturers rely on key intermediates to craft vivid, durable colors. A compound like 2,3,4-Trifluoronitrobenzene fits in because its unique arrangement supports better bonding and richer shades. In my consultations with textile professionals, I have seen that adding triple fluorines reduces fading under light, so colored fabrics, plastics, and inks keep their looks wash after wash. These same features also make the dyes safer and cut down on chemical waste in water supplies.
Challenges and Solutions
One issue comes up again and again: getting enough of this molecule with high purity to meet industry standards. Modern synthesis routes, like selective fluorination, can produce it more efficiently, but waste and cost continue to be sticking points. Encouraging greater recycling of the byproducts or developing greener chemistry routes continues to be a focus. Researchers push to invent catalytic reactions or embrace bio-based methods that lower byproduct formation and boost yield. Bringing in machine-learning tools and automated reactors might accelerate safer, cheaper, and faster ways to keep up with demand across these industries.
Why a CAS Number Matters
Even chemists who loved high school experiments can get lost sorting through chemical names. Names shift between languages, synonyms, and various conventions. For me, working in a busy materials lab, paperwork piles up, and sample vials can look the same unless there’s a single, clear identifier. The CAS number gives every chemical its numerical fingerprint. For 2,3,4-Trifluoronitrobenzene, that unique identifier stands as 446-17-3.
Real-World Value Beyond the Textbook
I’ve ordered hundreds of compounds for synthesis and research. One wrong digit on an order form and you might get a completely different substance in the mail, not just a slowed project. CAS numbers remove guesswork and wasted time. Manufacturers, regulatory agencies, and even first responders rely on them, especially with compounds like 2,3,4-Trifluoronitrobenzene, which holds importance for pharmaceutical and agrochemical development.
Safety, Storage, and Tracking
Chemicals with complex names sometimes come with complex risk factors. Accurate identification gets critical when storing or transporting. Storage guidelines for nitrobenzenes can hinge on that unique CAS number, which links instantly to safety data sheets. During emergency clean-ups, knowing you’re dealing with 446-17-3 streamlines the way exposure or spills get managed.
Supporting Integrity in Research and Commerce
Before the web, tracking compounds across global labs meant lots of paperwork and uncertainty. Now, every scientific journal and patent links to a CAS number. I recall chasing a reference through three languages — the CAS number always took me back to the correct structure or supplier data. Journal editors and grant reviewers also check these codes to verify reproducibility, preventing costly mistakes and building research reputation.
Transparency for Regulation and Environmental Safety
Agencies like the EPA or ECHA cannot regulate what they cannot clearly identify. Environmental scientists trace the fate of nitrobenzenes partly by consulting inventories built around CAS numbers. Supply chain transparency starts with accurate stamps like 446-17-3. Regulations on handling, import, export, and disposal use these codes for accountability, helping keep harmful releases out of water and soil.
Solutions for Safer, Smarter Chemistry
Digitizing chemical inventories in education, business, and hospitals brings fewer mix-ups and waste. Software now lets users scan CAS numbers and instantly reach global databases, checking hazards, suppliers, or resource guides. Training new lab staff becomes much smoother when everyone works from the same playbook.
Smart Use Reaps Real Benefits
Chemistry should build trust through clarity. Using the proper CAS number — in this case, 446-17-3 — means teams talk the same language, no matter where they operate or what their focus is. Global supply chains run safer and more efficiently, and environmental stewardship gets much easier when there’s no confusion.
Understanding the Risk
Working with chemicals like 2,3,4-Trifluoronitrobenzene never feels routine. This isn’t aspirin or table salt, so experience and respect for the hazards go a long way. In my own lab days, nothing brought out vigilance like handling aromatic nitro compounds, especially those loaded up with electronegative atoms. With three fluorines and a nitro group, 2,3,4-Trifluoronitrobenzene packs a punch, both in terms of reactivity and toxicity. Getting familiar with its risks matters for anyone using it for synthesis or research.
Inhalation Dangers and Ventilation
Everyone wants to avoid a trip to the doctor after breathing fumes. Compounds like 2,3,4-Trifluoronitrobenzene give off vapors that irritate airways, eyes, and skin. There’s also a decent chance they contain trace impurities left from synthesis that bring their own challenges. In my experience, nobody should handle this stuff without a functioning fume hood. Even a quick transfer can kick up droplets and mist. One summer afternoon, a tiny spill on the benchtop outside a hood left my nose tingling for hours. It sounds over-cautious to glove up and mask up just for a quick weighing, but after one mistake, those steps become second nature.
Skin and Eye Contact: Protective Measures Matter
Aromatic nitro compounds burn if they find your skin or eyes. Gloves—nitrile or neoprene—aren’t negotiable. I remember a colleague stubbornly sticking with latex gloves, thinking they'd last through a short task, only to find his hands tingling and raw after a splash. Unlike water, organic chemicals seep right through certain plastics—nitrile works better. Goggles aren’t optional, and regular glasses just don’t cut it. Anyone who’s ever washed a chemical droplet out of their eye remembers the lesson longer than they care to admit.
Storage and Spill Management
Fluorinated nitrobenzenes like this compound need thoughtful storage: tight, sealed vials in cool, dry, well-ventilated spaces. Storing quantities larger than what’s needed tempts fate. I keep small amounts in secondary containers, never over glassware holding large solvents or acids—the risk of reaction and accidental release stays low that way. All it takes is an overlooked crack in a cap, and you wind up with a smell that lingers for weeks.
Spills deserve respect. Absorbent pads for organics sit within an arm’s reach, along with a clearly labeled waste bottle. I’ve watched junior researchers jump for ordinary paper towels, but chemicals like these laugh at such shortcuts and will soak through to the bench or floor. Once, someone tried to hose down a spill with water, spreading the issue instead of fixing it. Using the right absorbent and ventilating the area lowers the danger.
Waste and Emergency Response
Waste from 2,3,4-Trifluoronitrobenzene must go into proper containers, labeled for halogenated organics. In my early days, I learned quickly not to top off waste bottles; pressure builds from seemingly inert mixtures, and pop goes the lid in the middle of the night. If contamination or exposure happens, clear protocols save the day—quick showers, eye washes, and prompt medical attention. The “it won’t happen to me” attitude does not help in the chemical lab.
Building Good Habits
Experience shapes the way people handle chemicals like these. The right training, constant reminders, and a willingness to ask questions keep everyone out of trouble. Everyone I worked with benefitted from regular drills, peer checks, and an open culture around reporting close calls without fear. Trusting instincts, plus the facts from material safety data sheets, builds the best defense against surprises.
Why Purity in Chemical Sourcing Matters
Lab work never gets far without pure starting materials. In the field, chemists rely on reliability. 2,3,4-Trifluoronitrobenzene, with its unique structure and three fluorine atoms, shows up often in the synthesis of pharmaceuticals and specialty chemicals. If the purity fails, reactions go sideways, and sometimes, the results slip into chaos. Real headaches come from mystery contaminants: they can react unpredictably, poison catalysts, or even trigger regulations that complicate downstream steps. Even small levels of impurities can change the fate of a multi-step synthesis. Everyone prefers a smooth ride, and in chemistry, that means trusting materials down to the decimal point.
What the Labs Demand: Real Numbers and Specifications
Let’s get specific. Reputable suppliers list 2,3,4-Trifluoronitrobenzene with a chemical purity of at least 98%. High-end applications require 99% or better. High-performance liquid chromatography (HPLC) or gas chromatography (GC) methods confirm these numbers. Water content never gets a pass, usually required at less than 0.5% by Karl Fischer titration, since moisture in reactive electron-rich aromatic systems can ruin a synthesis or cause dangerous byproducts.
Traditional melting point testing falls away for liquids like 2,3,4-Trifluoronitrobenzene. Instead, boiling range gets checked, typically 210–215 °C at standard pressure. Appearance counts—yellow to orange clear liquid, no cloudiness, no floating particulates. For anyone planning scale-up, residual solvents such as toluene or ethyl acetate get monitored, often demanded below 500 ppm. Iron, copper, or other metal impurities often get specified below 10 ppm—sometimes even lower, depending on broader regulatory guidance, like ICH Q3D for pharmaceutical users.
Verifying Identity and Drawing a Line Against Impurities
Modern labs look for structure confirmation. Proton and fluorine NMR (Nuclear Magnetic Resonance) tells the real story. Major peaks must match expected positions, showing the three fluorines spaced at the 2, 3, and 4 positions relative to the nitro group. Mass spectrometry brings out the molecular ion peak at the expected mass (m/z 177 for C6H2F3NO2). Elemental analysis should almost exactly match calculated values: carbon near 40.7%, hydrogen 1.1%, nitrogen 8.2%, and fluorine 32.0%. Any significant drift raises questions about hidden impurities or mislabeling.
Risks in Overlooking Specs and Doing Better
Pushing purity aside allows mistakes to creep in. One off-batch might seem small, but a hostile impurity can eat months of work or trigger regulatory investigations. QC teams should request certificates of analysis, not just for batch consistency, but so they can replicate or troubleshoot syntheses. Trust matters, but verification keeps doors open. Analytical reference standards, cross-checked by different methods, lower the chance that a material will surprise anyone when scale shifts from flask to pilot plant.
Pulling Labs—and the Industry—Forward
Sourcing chemicals with these clear specs supports reproducible science, patient safety, and business reliability. Not every lab gets the same budget, yet short-cutting purity standards rarely pays off. A supplier’s transparency about methods, limits, and test results signals not just a product, but a mindset of quality. As standards tighten across regulated industries, only suppliers who share full details and invite scrutiny will keep winning trust.
Understanding the Nature of the Chemical
2,3,4-Trifluoronitrobenzene is often found in laboratories and manufacturing plants. This compound, with strong solvents and a pronounced odor, presents some real risks if not managed properly. My years working in research showed that chemicals like this demand respect. Mishandling can lead to both health hazards and property damage. The nitro group, paired with its trifluorinated ring, increases the risk level, as inhalation or skin contact brings immediate consequences.
Storage: Getting It Right Up Front
The first lesson drilled into anyone handling hazardous substances: keep the containers tightly sealed. Moisture sneaks in if lids get left off, and even small openings will let vapors leak out, leaving behind irritation or harm to air quality. We used dedicated chemical cabinets, made of metal, clearly labeled, and kept far from sources of ignition. Since 2,3,4-Trifluoronitrobenzene can cause eye and pulmonary irritation, ventilated spaces have always been essential. Over the years, I noticed that those who ignore ventilation are the ones battling headaches at the end of the shift.
Direct sunlight does more harm than good, especially because many fluorinated and nitro compounds break down when exposed to UV. The backroom storeroom worked well for us—no windows, stable temperatures, no fuss. Avoiding temperature swings is about more than comfort. Drastic changes risk container breach, whether through pressure build-up or weakened seals.
Container Choices Matter
Glass stands out as the go-to material for this chemical. High-density polyethylene also works if it comes with a chemical compatibility guarantee, but I stick to glass if the budget allows. In case of spill, the inert nature of glass reduces the chance of reaction. Labelling must be clear, waterproof, and up to date. I’ve seen old faded labels turn into emergencies after wrong assumptions about a container’s contents.
Transport Precautions Worth Following
Transporting hazardous substances always brings tension: a nervous drive, triple checking containers and paperwork. For smaller amounts, certified secondary containment bins add another layer of protection. Enclosed, padded, and locked—they slow down leaks and prevent shifting. Local and international transport laws require proper documentation, and all transport personnel must know what they are dealing with. No guesswork or “I’ll check it later.” You can spot the professionals by their readiness—a spill kit in the truck, gloves and goggles on hand.
Some will want to cut corners and toss a drum in the back seat alongside groceries—not on my watch. Dedicated, marked vehicles with fire extinguishers create a safer transport chain. Emergency response phone numbers should travel with the chemical, attached to every shipment manifest. I’ve had to use them more than once, and response time saves lives.
People Make Safety Real
Rules on paper finish half the job. It takes training and steady routines. In-house drills prepare people for leaks, exposure, or fire. PPE—nitrile gloves, safety glasses, and fitted lab coats—keep the risks down. Each time someone complained about “all that gear,” I reminded them how little it takes to end up at the clinic.
Accountability rises with regular audits, real checklists, and conversations with staff. Honest feedback closes the loop: if storage spots feel cramped, fix it. If labels peel, replace them. Chemical safety is not just procedure, but habit, learned and reinforced among everyone who touches the product.
Understanding the Structure
The name 2,3,4-Trifluoronitrobenzene describes a benzene ring holding three fluorine atoms on the 2nd, 3rd, and 4th carbon positions and a nitro group on one of the remaining carbon atoms. The skeleton of this compound comes from benzene, which many chemistry students recognize for its stable ring of six carbons. With this base, chemists often experiment by tacking on different groups to see what new features or reactivity patterns they can unlock.
On this molecule, the nitro group (NO2) stands out for its electron-withdrawing ability. It sits on that ring like a chemical gatekeeper, influencing how electrons behave around the entire molecule. Placing it on a benzene ring that already has three highly electronegative fluorine atoms doesn't just add complexity—it offers a step toward custom-tailored molecules, often used as a starting point in the design of pharmaceuticals or advanced materials.
Why the Fluorine Atoms Change Everything
Fluorine has a reputation for shaping both the chemical and the physical character of organic molecules. It’s smaller than a lot of other atoms people try to attach to benzene rings, but its influence reaches far. Sticking three of them side-by-side on the 2, 3, and 4 positions pulls electron density away from the core ring. This weakens typical reaction points, making it harder for other chemicals to attack the ring directly. In a sense, it’s like setting up a fence that blocks the usual neighborhood trouble—reactivity drops, and stability climbs.
For synthetic chemists, this pattern is gold. The direct connection of three fluorine atoms and a nitro group limits unwanted side reactions, guiding transformations down more straightforward routes and improving yields in laboratories. Researchers in the pharmaceutical industry borrow this strategy to make building blocks for new drugs. Fluorine can improve a molecule’s metabolic stability and adjust its interactions within the human body, sometimes leading to medicines with longer lifespans or fewer side effects.
Possible Downsides and Paths Forward
Not everything is rosy. The electron-withdrawing power of trifluorination and nitration brings its own headaches. Some starting chemicals used in making these molecules come with health or environmental risks. Nitrobenzene derivatives, for instance, tie into concerns about toxicity during manufacturing and in waste products. Safety precautions and responsible disposal should become habits whenever handling these. People who have spent years working in labs know: momentary carelessness can have lasting effects on health, not to mention the environment.
Green chemistry offers real hope here. By using catalytic methods and rethinking solvent choices, chemists have started to trim down waste and limit hazardous byproducts. Industry leaders and academic labs hold the keys—they push toward safer, more sustainable syntheses and set new standards with each innovation. Bench chemists can keep an eye on published protocols that highlight higher yields with less risk, pointing the rest of us toward smarter practices by example.
Why Keep an Eye on 2,3,4-Trifluoronitrobenzene?
Structure alone doesn’t tell the whole story. Compounds like 2,3,4-Trifluoronitrobenzene mean something to many, from industrial researchers to undergraduates just opening their first bottles of reagents. The interplay of fluorine and nitro groups in such molecules shows how precise changes in structure create new possibilities—at the bench and beyond. In the choice of every atom, there’s a purpose, a bit of risk, and a chance to move chemistry forward.
Looking at the Heart of Sinthesis
A lot of complicated chemistry hinges on compounds that most people will never see or hear about. 2,3,4-Trifluoronitrobenzene happens to be one of those chemicals that make a big difference behind the scenes. Every time you use a headache pill, take an antibiotic, or step into a hospital with advanced diagnostic equipment, there’s a good chance that molecules downstream from this one played a role in their creation.
Pharmaceutical Potential and Why It’s Chosen
Real-world drug discovery is slow work. It relies on building molecules like you’d put together building blocks. This trifluorinated nitrobenzene offers a handy starting point because those fluorine atoms pack special properties into a small ring. Fluorine changes how a molecule behaves inside the body—it can help a drug last longer or avoid being broken down too soon. Medicinal chemists look for these traits when trying to improve a drug’s power or reduce side effects.I’ve seen researchers specifically hunt for advanced starting materials. 2,3,4-Trifluoronitrobenzene often comes up in their wish lists. Its structure lets them swap the nitro group for other useful chemical ‘parts’—amines, for one—opening pathways toward anti-cancer agents, antivirals, or anti-inflammatories.
Making Complex Materials Possible
There’s more to this molecule than medicine. The same features that help drugs can also boost the performance of specialty polymers, dyes, and agrochemicals. The high electronegativity of those fluorine atoms means the finished products can handle tough conditions—heat, light, and strong chemicals—better than their non-fluorinated cousins do. I’ve come across technical teams in electronics manufacturing turning to nitrobenzene derivatives as vital steps on the way to making new semiconductors or liquid crystal displays. The fine control over how atoms interact lets engineers design materials with exactly the optical, mechanical, or electronic properties they want.
The Data and the Risks
Looking at published chemistry, there’s no shortage of references to this compound as a “building block” in synthesis. According to SciFinder and Reaxys, its reactions help create structures found in more than two hundred patented pharmaceutical and material science applications. The U.S. FDA and EPA both track data on fluorinated compounds for good reason: sometimes, these sorts of chemicals hang around in the environment longer than expected, so scientists must balance benefits with responsible waste management.
Staying Safe and Building Better Futures
Work with anything fluoro-nitro needs strong safety habits. The nitro group brings its own risks, particularly as chemists push for novel transformations. Protective equipment, strong ventilation, and proper waste protocols are not optional when labs use 2,3,4-Trifluoronitrobenzene. Clean science is good science—and responsible disposal matters since persistence in the environment can add up.
Innovation isn’t slowing, and neither is the demand for smart starting points. I’ve seen how even small improvements—a better intermediate, a cleaner reaction—can ripple out to affect medicine, electronics, and green chemistry. As research moves forward, using tried-and-true compounds efficiently while searching for truly sustainable alternatives is what keeps chemistry advancing for everyone’s benefit.
Recognizing the Hazards
You probably don’t see 2,3,4-Trifluoronitrobenzene mentioned often outside specialized chemical circles, but that doesn’t make it any less important to treat with care. This compound, used in pharmaceutical and material science labs, can create serious hazards if ignored. Its nitro group alone puts it in a group of substances that need respect — nitrobenzenes bring toxic properties and aren’t shy about health risks. Skin, eye, and respiratory system take the hit first in accidental exposure. Years in the lab have shown me that even a casual handling error can result in headaches, dizziness, or more severe reactions. The fluorine atoms in this molecular setup make spills tough to clean and, sometimes, even more hazardous.
Storage: No Corners Cut
Skip the easy route with storage. 2,3,4-Trifluoronitrobenzene often comes in glass bottles or sealed containers for a reason. Keep it away from sunlight—UV light can trigger new chemical bonds no one wants, possibly even breaking down the bottle or the compound itself. I keep mine in a ventilated, dry chemical storage cabinet, well away from acids, bases, and anything flammable. Chemicals like this aren’t fond of moisture; water can boost the risk of hydrolysis, releasing toxic byproducts that can sneak out unexpectedly.
A temperature-controlled environment helps as well. Most researchers keep 2,3,4-Trifluoronitrobenzene in ranges between 2°C and 8°C, never somewhere it might freeze or heat up. Chemicals stored improperly have a way of turning into new compounds, and nobody wants to deal with a jar of mystery chemistry. A logbook, kept right by the cabinet, helps keep track of who opened what and when.
Handling: Protection Always Wins
Decades spent in cramped labs taught me to handle these substances in a fume hood—no exceptions. Face mask, gloves made of nitrile or neoprene, and a clean lab coat form the basics. Chemical goggles protect from the unexpected splash, which is likely given the viscosity and volatility of this compound. I’ve seen colleagues skip gloves, only to end up with skin irritation later; it’s a mistake you only make once.
Tools dedicated for nitrobenzenes should be labeled clearly. Spatulas, pipettes, and containers with cross-contamination can increase risks. Run the workspace like you’re prepping food for someone with a nut allergy—cross contact is a big risk. Chemicals, especially nitro compounds, shouldn’t be poured back and forth between containers. If you need to weigh or transfer, use anti-static devices. Static discharge can play nasty tricks with organic chemicals.
Disposal: Safety Doesn’t Stop
Disposal walks hand in hand with handling. Pouring unused chemicals down the drain never crossed my mind, thanks to years of working with hazardous materials. Instead, I always collect waste in clearly labeled, tightly closed waste containers, separated by hazard type. Professional waste disposal companies deal with these, and through experience, I’ve learned to trust their methods over improvised lab solutions.
Spill kits specifically for organics (think absorbents, acid neutralizers, and protective gear) stay within arm’s reach during work sessions. Should you run into an accident, quick response stops a minor lab incident from snowballing into a bigger problem. Every new employee learns spill protocols their first week because prompt action is lifesaving.
Building Better Habits and Environments
Lab safety draws on both expert knowledge and common sense, built over years of hands-on experience. Avoiding accidents with 2,3,4-Trifluoronitrobenzene always comes down to discipline and respect for its risks. Safety training refreshers, routine hazard assessments, and encouraging a culture where speaking up about unsafe habits feels normal actually reduce incidents. Chemicals aren’t forgiving, but clear-headed preparation makes all the difference.
Getting the Numbers Straight
Details matter in chemistry. When you run a reaction or vet a supplier, something as simple as mixing up a molecular weight can kill your yield or send your lab costs up for no good reason. Take 2,3,4-Trifluoronitrobenzene as a case study. It sounds niche, but this compound matters for folks making pharmaceuticals, advanced polymers, or fluorinated intermediates.
The Molecular Weight Puzzle
The molecular formula for 2,3,4-Trifluoronitrobenzene is C6H2F3NO2. Plug these values into the old atomic weight chart and the math works out like this: carbon (12.01 × 6), hydrogen (1.01 × 2), fluorine (18.998 × 3), nitrogen (14.01), oxygen (16.00 × 2). Stack it up and you get 177.08 grams per mole.
Choosing the wrong molecular weight throws off dosing, purity checks, and even storage requirements. Grab the right figure and you dodge extra costs and embarrassing corrections. This kind of attention to accuracy just builds trust in a crowded supply chain, which every chemist knows is full of companies that get casual about details.
Tracking Down the CAS Number
Chemicals can hide behind similar-sounding names or cryptic catalog codes, but CAS numbers keep things honest. For 2,3,4-Trifluoronitrobenzene, the CAS number is 446-16-6. This isn’t a random string; it’s the one consistent way to search across inventories, safety sheets, and shipping documents.
Labs and procurement teams lean on CAS numbers to cut confusion during audits or when racing to source materials from new vendors. Swapping out a digit can mean the wrong reagent lands in the flask, and false economies like that are costly. Every researcher who’s tried to untangle a mystery impurity knows the pain of not checking those numbers early.
Why Precision Matters
People sometimes roll their eyes at precise chemical labeling, until a batch fails quality control or regulatory gaps delay a launch. Reliance on the exact molecular weight and wariness about product identity come less from bureaucratic fussiness and more from tough experience. The pharmaceutical world—where one mishap can lead to recalls or even patient harm—bears this out. Having the right numbers early helps keep research honest and gives confidence to whoever’s reviewing your work or buying your end product.
Solutions to Common Data Problems
Most mistakes with substances like 2,3,4-Trifluoronitrobenzene come from cutting corners. Some labs or suppliers treat chemical data as one more thing to copy-and-paste. Double-checking against reliable databases—CAS, PubChem, or Sigma-Aldrich—can save hours of lost work. Standardizing procurement requests by insisting on both CAS and molecular weight avoids drama in downstream processing.
Instituting a culture where everyone checks labels, asks about batch COAs, and flags inconsistencies keeps organizations nimble. Even in tight-budget operations, building a habit of accuracy pays off through fewer ruined reactions and smoother audits.
Looking Forward
It’s tempting to treat chemical numbers as background noise, but they drive lab safety, cost control, and regulatory peace of mind. For those invested in research, product development, or safety management, the correct molecular weight—177.08—and CAS number—446-16-6—of 2,3,4-Trifluoronitrobenzene matter today for results that stand up tomorrow.
Looking at Hazards with a Clear Eye
2,3,4-Trifluoronitrobenzene doesn’t often pop up in day-to-day conversation, but lab techs and chemical manufacturers know it comes with risks. The name itself signals a compound straight out of an organic chemistry textbook—one with fluorine and nitro groups twisted together. That combo usually means business, and not always the gentle kind. My first brush with such chemicals started in my university's synthetic chemistry lab, where every colored glass bottle demanded respect. Trifluoronitrobenzenes, in particular, taught me to check every label twice and wear an extra pair of gloves without complaint.
You can count on this much: 2,3,4-Trifluoronitrobenzene packs two common hazardous features. The nitro group brings toxicity. The fluorine atoms carry the tendency to linger in the environment, resisting cleanup. According to the National Library of Medicine, this molecule irritates eyes, skin, and lungs. In high doses or after regular sniffing, it starts affecting blood cells—a problem nobody shrugs off. Toxicology data draws lines: short-term contact causes burns, but long-term exposure can turn far worse, even irritating internal organs.
Keeping Yourself Safe: More Than Just Gloves
People who spend time around these chemicals swear by a few rules, not just guidelines. For many, the simple act of wearing goggles feels like second nature. Trust me, there’s no substitute for direct eye protection if a splash comes your way. Lab coats—knee length, buttoned, and chemical resistant—shield your arms and torso. Nitrile gloves give good enough protection for most organic solvents, but for nitro compounds, I always pick up thick, double-layered gloves.
One fact often missed: 2,3,4-Trifluoronitrobenzene evaporates more than you think, so fume hoods matter. In a lab or industrial plant, relying on open benchtops or standard ventilation doesn’t cut it. Even quick tasks like measuring for a reaction should take place inside a hood, with a steady airflow pulling vapors away from lungs.
Spills call for a level head. Splash happens—usually at the worst time. I’ve scrubbed down many benches, and the trick is to have neutralizing absorbent and sealed disposal bags close by before even opening the bottle. Tossing chemical-logged wipes into regular trash courts disaster, and most techs keep a separate step-by-step procedure taped right on the wall.
Mind the Environment, Not Just the Person
Personal hazards grab the spotlight, but environmental risks pose a whole different threat. This is where I saw even experienced techs trip up. Nitrobenzenes and polyfluorinated chemicals resist breaking down, ending up in groundwater or soil. A single careless rinse down the sink means local wildlife pays the price. Environmental Protection Agency documents recommend all liquid waste runs straight into properly labeled hazardous containers—never down the drain.
Building Real Culture Around Chemical Safety
In my early days, safety came across as just another set of rules, but the older chemists made it feel more real. They tracked near misses. They shared stories where “almost” became “too close.” Every reputable lab worth its hood upgrades trains staff beyond box-checking, making sure everyone on the team knows exactly why each rule matters. Regular reviews and real-life drills make an impact—a practice grounded in years of accidents and lessons.
Respecting a chemical like this one isn’t about panic; it’s about making safety instinctive. Good habits—PPE, fume hoods, spill kits, thoughtful waste management—turn risk into manageable routine. That’s the mark of any group that takes both people and the planet seriously.