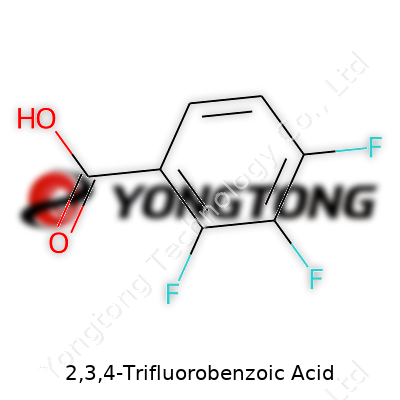
2,3,4-Trifluorobenzoic Acid: Deep Dive into a Key Fluorinated Intermediate
Historical Development
Scientists didn’t always pay much attention to trifluorinated benzoic acids. During the rise of organofluorine chemistry in the mid-20th century, researchers working to develop new agrochemicals and pharmaceuticals started exploring selective trifluorination of simple aromatic compounds. The 1970s and 1980s saw a significant jump in industrial interest, as methods for controlled fluorination matured. Early syntheses were fraught with low yields, trace impurities, and dangerous reagents. Modern approaches, refined by decades of R&D, make manufacturing more efficient and a lot safer. Many synthetic chemists now recognize 2,3,4-trifluorobenzoic acid as a key intermediate with growing significance as fine chemicals, advanced polymers, and active pharmaceutical ingredients branch into more complex designs.
Product Overview
2,3,4-Trifluorobenzoic acid offers more than just a catchy name among fluorinated aromatics. By adding fluorine atoms to the aromatic core, properties shift—higher lipophilicity, better metabolic stability, and a distinct reactivity profile compared with its non-fluorinated cousin. Compound suppliers now list it for everything from drug research to custom materials. Purities typically reach above 97% for most industrial and laboratory uses, underscoring its role as a reliable building block. Demand comes from both innovators mapping out new synthesis routes, and large manufacturers scaling up fine chemicals for end uses like crop protection and electronics.
Physical & Chemical Properties
2,3,4-Trifluorobenzoic acid forms a white or off-white crystalline powder, and remains free flowing under standard storage. Its melting point rests near 163–166°C—higher than that of benzoic acid itself, due to fluorine’s influence. The compound dissolves sparingly in water, but shows higher solubility in organic solvents like acetone, ethanol, and DMSO. Low volatility means minimal vapor hazards during transfers and handling. Chemical stability is strong, even at elevated temperatures, unless exposed to strong bases or reactive reducing agents that can disrupt the aromatic ring. Analytical fingerprints from NMR and IR line up exactly where chemists expect, confirming the rigid trifluorinated structure and guiding process QC for every batch.
Technical Specifications & Labeling
Suppliers selling to research centers and industrial clients usually stamp technical sheets with comprehensive specs: molecular formula C7H3F3O2, CAS number 446-17-3, and purity guaranteed above 97–98%. Testing by HPLC confirms purity while GC-MS or NMR screens for trace contaminants and isotopic variants. Labels display batch codes, hazard symbols, risk phrases, and storage recommendations—room temperature, away from moisture and strong oxidants. Certificates of analysis (COA) arrive with each shipment, meeting regulatory and quality standards imposed by both customers and authorities like REACH or the US EPA for environmental tracking.
Preparation Method
Commercial processes for 2,3,4-trifluorobenzoic acid build off years of fine-tuned chemistry. The classic route involves selective electrophilic fluorination of a substituted benzoic acid, using reagents like Selectfluor or elemental fluorine under strict controls. Some routes harness Suzuki or Ullmann couplings, using trifluorinated phenylboronic acids as key intermediates, joined in the presence of palladium or copper catalysts. Isolation usually calls for acidification of the reaction mixture, extraction with an organic solvent, solvent evaporation, and recrystallization. Yields have climbed as new catalysts and milder conditions substitute for old, hazardous approaches—making production safer and more cost-effective for both small-batch specialists and kilo-lab manufacturers.
Chemical Reactions & Modifications
The chemical personality of 2,3,4-trifluorobenzoic acid opens doors to further transformation. The carboxylic group handles esterification, amidation, and reduction under typical organic conditions. Many medicinal chemists use it as a starting point to craft fluorinated benzamides, esters, or even more complex heterocyclic scaffolds. The aromatic ring, now less reactive due to the three electron-withdrawing fluorines, resists most electrophilic substitution—giving synthetic planners a chance to work around the aromatic system with high confidence in product selectivity. Industrial groups chasing specialty polymers or fluorous surfactants often attach bulky or functional groups onto either the carboxyl moiety or the ring, using the parent acid for anchoring diverse new structures.
Synonyms & Product Names
Catalogs might list 2,3,4-trifluorobenzoic acid under several alternate names: 2,3,4-Trifluorobenzoate, Benzoic acid, 2,3,4-trifluoro-, or NSC 162193. Globally, suppliers tailor packaging and product codes for easy tracking in chemical databases. These synonyms help researchers and supply chain managers cross-reference compound lists, avoid confusion with isomers, and ensure correct ordering for complex formulation or scale-up projects.
Safety & Operational Standards
Every lab worker and scale-up technician keeps safety protocols close at hand with 2,3,4-trifluorobenzoic acid like any specialty reagent. The compound’s low volatility limits inhalation risk, but dust control remains key. Direct skin or eye contact causes moderate irritation, so nitrile gloves and protective eyewear go on before weighing or transfers. Facilities monitor air quality and ensure spill procedures match global chemical safety standards. Waste handling must follow environmental protection rules—neutralize acidic residues, then collect aqueous and organic wastes according to local hazardous waste regulation. Safety Data Sheets (SDS) supplied with each order sight rugged, clear guidelines for storage, incident response, and first aid.
Application Area
2,3,4-Trifluorobenzoic acid steps up in quite a few chemical sectors. Drug discovery teams use it for new fluorinated analogues with enhanced metabolic stability, hoping to boost performance against rapid breakdown in the body. Agrochemical designers slot it into pesticide and herbicide core structures, seeking increased potency and longer field lifespans. Materials scientists look at the acid for advanced fluorinated monomers, powerful enough to impart water, oil, or stain resistance to consumer products and industrial coatings. Dye manufacturers sometimes turn to it for special-effect pigments with unique fluorescence. Academic researchers, in both undergraduate teaching and cutting-edge fluorous chemistry, seek out 2,3,4-trifluorobenzoic acid to explore aromatic substitution and influence of fluorine atoms on chemical systems.
Research & Development
Teams working in R&D often find that adding three fluorine atoms to a simple benzoic acid flips the script on reactivity and bioactivity. Multiple research groups are now investigating new synthetic routes to increase atom economy, reduce hazardous byproducts, and lower energy input. Medicinal chemists run structure-activity relationship (SAR) studies, cycling through trifluorobenzoyl building blocks hoping to identify next-generation therapies for cancer, neurological, or infectious diseases. On the academic side, recent work also digs into supramolecular chemistry and catalysis, harnessing trifluorinated acids to tune the acidity or hydrogen-bonding profile of custom-designed host molecules. Many companies have private patent applications or protected processes built on the backbone of 2,3,4-trifluorobenzoic acid.
Toxicity Research
Assessment of toxicological risks has become a central concern, especially as fluoroaromatics attract attention for bioaccumulation. Early animal studies put its oral LD50 higher than many industrial acids—yet ingestion, inhalation, or skin absorption in significant quantities still brings real harm. Chronic exposure studies track bioavailability and possible interference with metabolic enzymes. Regulators take a cautious approach, especially for environmental discharge and trace contamination in water streams: local rules force factories to run regular analytics for fluorinated byproducts and strictly limit allowable concentrations. Responsible suppliers update toxicity research, harmonize with OECD and GHS guidelines, and supply clients with the latest findings on safe exposure windows and proper PPE for handling the acid in any setting.
Future Prospects
Looking forward, utility of 2,3,4-trifluorobenzoic acid stands poised to expand rapidly. Breakthroughs in sustainable fluorination, such as electrochemical and biocatalytic routes, promise reduced environmental impact and lower costs for mass production. Chemical engineers focus on closed-loop recycling for fluorinated intermediates, shrinking hazardous waste streams and capturing value from otherwise lost byproducts. Drug developers see fluorinated benzoic acids as cornerstones for designing more resilient, longer-lasting molecules, and material scientists push for new blends that combine high performance with lower perfluorinated emissions. Regulatory pressure will likely ramp up, driving more transparency and innovation toward safer, greener processes. The next decade could see expanded application for 2,3,4-trifluorobenzoic acid, riding the wave of global demand for smart materials, novel crop protection, and next-generation medicines—all pivoting off a small but powerful fluorinated ring.
Looking at the Atoms: What’s Really There
2,3,4-Trifluorobenzoic acid stands out in the world of organic chemistry because it puts three fluorine atoms into play, attaching them right onto the benzene ring. Imagine a standard benzoic acid—there’s a benzene ring with a carboxylic acid group (-COOH) at the number one spot. Now, drop a fluorine atom onto the second, a second fluorine on the third, and a third at the fourth carbon on that ring. That setup carves out the core structure of 2,3,4-Trifluorobenzoic acid.
The chemical formula usually gets written as C7H3F3O2. You’ll see those three hydrogens replaced by fluorines at consecutive places on the six-carbon benzene backbone. Each fluorine acts differently because of its strong pull on electrons. That changes the whole game for how this molecule acts in a reaction or in the body.
Why Fluorines Change the Game
Adding fluorine is never just a swap in the textbook. Fluorine atoms electrify a molecule. Chemists see the changes in reactivity, acidity, and even how the molecule fits into biological spaces. For benzoic acids, swapping in fluorine near the acid group sharpens the acid’s edge. Fluorine tugs at the electrons and makes the hydrogen on the -COOH more likely to leave, cranking up acidity.
From experience in the chemistry lab, I know how swapping atoms transforms more than just numbers on paper—it shifts solubility and changes how the compound travels through water or fat. All three fluorines across the 2, 3, and 4 spots work together to pull electrons toward themselves. The electron cloud in the ring ends up pretty lopsided, and that makes the carboxylic acid behave differently from its non-fluorinated cousin.
Applications: Tracking Structure to Use
In drug discovery, sticking fluorine on a molecule often makes a candidate more stubborn against being broken down. Medicines with these traits can stick around long enough to get the job done. Trifluorinated benzoic acids jump into research focused on anti-inflammatory drugs, crop protection, or as building blocks for more exotic chemistry. The structure helps make sure these molecules resist enzymes that would otherwise chew up drugs too fast.
Industry relies on specific chemical setups to get the strength, resistance, or reactivity needed. I’ve seen trifluorinated compounds used where you want selective reactivity: the molecule clings to the target, but shrugs off much of everything else. That performance really starts with those three fluorine atoms in their particular places on the ring.
Challenges in Handling and the Path Forward
All those electronegative fluorines may boost usefulness, but they can also raise challenges in waste management and safety. Fluorinated compounds stick around in the environment longer, and breaking them down safely requires careful planning. From the bench to the factory, this means taking extra precautions, developing better routes that use fewer harsh reagents, and investing in research for greener chemistry. Solutions start from designing synthesis methods that minimize leftovers, then figuring out how to recycle or safely neutralize anything left behind.
Better knowledge about molecular structure can lead not just to more efficient processes, but also to safer labs and a cleaner world. This only happens with clear eyes on the atoms and respect for the way they shape what a compound can do—and what problems it might bring along, too.
Everyday Chemistry That Shapes High-Tech Fields
2,3,4-Trifluorobenzoic acid doesn’t get public attention like the big headline compounds do, but it's quietly doing a lot behind the scenes. This fluorinated benzoic acid pops up in laboratories, on production floors, and even in places that impact our daily health. Some years back, I learned how adding just a couple of fluorine atoms to a compound could totally change its personality—sometimes, that turns an ordinary molecule into a key player.
Building Blocks for Medicine and Discovery
In drug development labs, small changes add up. Drug molecules often need fluorine for stability or for controlling their interactions inside the body. 2,3,4-Trifluorobenzoic acid provides those specific fluorine groups that medicinal chemists crave. By linking up this acid with other parts, researchers build trial drugs that stick around longer in the body or dodge breakdown by our enzymes. Take a look at current pharmaceutical research; fluorine substitutions like this one often show up on promising new therapies, since they help control absorption, distribution, and how the drug acts. Fluorinated building blocks also allow scientists to probe exactly how drugs work, fitting like puzzle pieces into bigger molecules.
Crop Protection: Supporting Global Food Security
Agricultural labs often look to fluorinated compounds to strengthen the arsenal against pests and blights. Compounds derived from 2,3,4-Trifluorobenzoic acid make crops less vulnerable because these molecules stand up well to sun, rain, and stubborn fungi. The acid acts as a base point for new herbicides or fungicides. Because of its reactivity, it helps chemists design molecules that disrupt pests’ lifecycles without breaking down too quickly in the environment. With global populations putting pressure on food supply, chemistries that protect yield and lower waste are too important to ignore.
Materials with a High-Tech Edge
Electronics and materials scientists lean on tough, stable molecules for new products, and this acid checks the right boxes. Building advanced polymers often relies on putting fluorine in precise spots. Fluorinated materials can resist heat or chemical spills much better than their non-fluorinated cousins. For example, think about the coatings on wires or in microchips, places where a short circuit or chemical leak could prove catastrophic. The strong carbon-fluorine bonds in molecules like this acid deliver that extra margin of safety.
Looking Forward: Sustainability and Safety
These uses sound great, but experience tells me there’s always a flip side. Some fluorinated chemicals hang around in nature much longer than we want, and getting them back out isn’t easy. Innovators have taken note, using this acid to build molecules flexible enough to break down when needed, or picking methods that lower chemical waste. Researchers now test for environmental impact much earlier in development. Tightening safety and waste regulations pushes the whole industry to work smarter. Finding cleaner production methods for compounds like this—using renewable feedstocks or smarter catalysts—makes for better chemistry, less pollution, and a safer workplace for all.
The Realities of Safeguarding Chemicals
Working with chemicals like 2,3,4-Trifluorobenzoic Acid demands smart handling. Even in small labs, one careless move could turn a smooth routine into a headache. This compound brings its own quirks—white crystalline powder, very mildly aromatic, not dusting up easily but still able to irritate skin or eyes. It isn’t enough just to put the jar on a shelf. If the storage space is wrong or labeling slips, the stakes jump from minor hassle to emergency.
Why Tackling Moisture and Heat Are Non-Negotiable
2,3,4-Trifluorobenzoic Acid keeps stable at room temperature, which can lull people into treating it casually. In reality, its longevity depends on a clean, dry, cool environment. Moisture invites clumping or slow breakdown. High humidity can even nudge unwanted reactions if it sits unchecked for weeks. Leaving the container open or carelessly recapping after every scoop helps nothing. Desiccant packs aren’t just a box decoration—they’re a lifeline for quality.
Practical Steps That Actually Work
My early days in chemical handling showed me the cost of skipping basics. Now, every time I see a bottle of 2,3,4-Trifluorobenzoic Acid, I check if it’s kept in a tightly sealed glass or HDPE container. I avoid storing it anywhere near strong acids, bases, or active metals. This isn’t just lab protocol—it keeps cross-contamination or unexpected reactions from happening. Proper labeling isn’t just a rule—labels often get worn or faded, so regular checks help keep confusion or mistakes at bay.
Storing it away from sunlight and direct heat keeps its chemical structure consistent. Shelves should feel cool—not freezing, but never above 25°C. Before I wrap up for the day, I always check the workbench for leftover spills. Even trace residue on gloves or glassware can travel fast and surprise you later. Good personal protective equipment (PPE) isn’t for show—goggles, lab coats, and gloves set a clear boundary between skin and substance. Simple routines like this build good habits that stick.
Waste and Emergency Planning: Not Just a Paper Checklist
Disposal often gets ignored until a bottle runs out or someone smells something off. Don’t dump 2,3,4-Trifluorobenzoic Acid or its waste down the drain—it counts as hazardous. Seal it in a compatible, clearly labeled waste container and schedule pickups through a licensed provider. Spills should trigger an immediate response: ventilate the area, sweep up powder with care, and never raise dust. Anything contaminated with the acid goes in sealable poly bags that wait for proper disposal, not the regular trash.
Relying on good training pays off. If someone starts coughing or complains of eye irritation, you want clear signage for safety showers and eyewash stations. Quick access to Safety Data Sheets makes the difference when every minute counts. These are simple investments—time, vigilance, and respect for chemistry go a lot further than flashy equipment.
Better Habits Lead to Safer Labs
Building a culture that values tidy storage and careful handling benefits everyone. Keep chemicals—2,3,4-Trifluorobenzoic Acid included—out of high-traffic zones. Regular audits help spot missing labels, leaky lids, or misplaced containers before problems grow. Storage and handling boil down to respect—for the material, the people in the lab, and the science itself. Every small step away from carelessness sets a better standard for everyone who follows.
Why Purity Shapes Quality and Trust
Buyers want to know exactly what they’re getting. Purity isn’t just a lab number—it means you have a clear idea of how much of the product is actually the material advertised. In today’s market, purity levels aren’t just fluff for the product spec sheet. High purity brings peace of mind and helps businesses avoid unwanted surprises. If I’m sourcing chemicals or ingredients, every fraction of a percent matters because contamination or impurity can throw off an entire process or recipe.
Take the food industry, for example. If a bulk ingredient like citric acid contains trace impurities, the end product could taste different or even break safety regulations. In electronics manufacturing, just a bit of the wrong element in a batch of silicon or copper will turn a promising device into scrap. A 99.9% labeling isn't much comfort if your specific use demands 99.99%: that tenth of a percent can make or break a batch. Companies now list purity levels to the decimal because customers have learned to demand full transparency.
For most raw materials, high purity reduces risks down the line. Production interruptions cost time and money. Delivery of off-spec product isn’t just wasteful—it can damage business relationships. I’ve learned to ask for documentation on purity, such as certificates of analysis from third-party labs. This isn’t about a lack of trust, but about ensuring everyone plays by the same clear rules.
Packaging Sizes: Meeting Real-World Demands
Suppliers like to offer several packaging sizes to suit different buyers. A large factory might order by the ton, while a research team could need just a kilogram. For a bakery or small manufacturer, giant drums sitting on the dock quickly become a storage headache. Smaller containers cost more per unit, but help avoid waste and keep product fresh.
I’ve seen firsthand how choosing the wrong size causes problems. Once, a client bought a solvent in 200-liter drums, only to realize their work area could only safely hold 20-liter cans. Their staff ended up decanting product by hand, which created safety risks and spilled a fair bit of expensive material. Packaging matters almost as much as what’s inside.
Packaging also hints at how a supplier treats their customers. If a distributor offers only huge bulk sizes, they’re often thinking about their largest buyers. Those who also supply small packs show they’re willing to support niche users and small businesses. That flexibility builds long-term relationships rather than just quick sales.
Building Confidence Through Information
Supply chain mishaps cause enough stress without guesswork about purity or packaging. Buyers trust suppliers to lay out these facts plainly. EU and US regulations pressure companies to disclose content and packaging information clearly, so reputable suppliers put these details right on their product pages or spec sheets. If details are missing, I always recommend a direct phone call or email—the extra time pays off in fewer surprises later.
Transparency makes it easier for everyone to plan purchases, keep projects on schedule, and avoid unnecessary risk. A supplier’s willingness to share precise purity figures and practical packaging options shows they have experience and understand everyday problems in business and industry. That’s the kind of partnership customers want, because it saves time, money, and hassle down the line.
Getting to Know the Hazards
2,3,4-Trifluorobenzoic acid isn’t a chemical most folks see often unless they work in a lab or chemical plant. As an organic compound with three fluorine atoms sitting on a benzoic acid ring, it pops up in research circles. A few years of hands-on work in chemical supply showed me that even what seems like a plain powder demands respect. Overlooking small details leads to accidents, no matter how common the chemical.
Fluorinated acids have a certain bite. This acid can irritate the skin, eyes, and nose. Direct contact stings – no better way to put it. Inhalation of the dust leads to coughing and sometimes headaches or worse: respiratory issues. Whether one pellet or a cloud of powder, contact should be taken seriously. More than once, I’ve seen new lab techs brushing powder off their gloves without thinking. Exposed skin feels it quickly, even if the irritation seems mild at first. Eye exposure tends to be very painful; on one occasion, a coworker forgot their safety glasses and paid for it with a trip to the eyewash station.
Thinking Beyond Simple Labels
Sometimes, labels only mention “irritant.” This doesn’t do justice to the repeated or long-term results of exposure. Trifluorobenzoic acid can lead to dermatitis where the skin gets red and starts peeling with ongoing contact. People with a tendency to allergies might react even more strongly. Swallowing even small amounts leads to chemical burns along the throat and stomach lining, which sounds rare, but accidents do happen in busy labs or production spaces. Just last year, a minor spill on a benchtop in my building led to accidental contamination during lunch break – a good reminder that compartmentalizing work and eating areas prevents risky slipups.
Fluorinated compounds, as a group, sometimes break down into toxic gases when exposed to strong acids or high heat. Heating trifluorobenzoic acid past its melting point in a poorly ventilated room isn’t just uncomfortable; it spins up fumes that can do real harm. Small spills on hot plates sometimes give off an odd, irritating odor that signals a bigger air quality problem. Opening a window doesn’t always solve the issue; real ventilation is needed.
Safe Handling and Solid Habits
I’ve seen the impact of good habits. Goggles and nitrile gloves should become second nature with this compound. Changing gloves after handling avoids surprise exposure for coworkers. Clothes covering all exposed skin cut down on irritation, and keeping a dedicated dust mask or respirator stops accidental inhalation. Chemical fume hoods change the game, pulling bad air away before harm sets in.
Emergency readiness matters more than company policies or posted placards. Knowing where eyewash stations and showers sit helps in those seconds after a splash. A monthly drill makes more sense than neat paperwork. I remember the difference between two teams: one kept a written manual, the other drilled for emergencies. The latter never had lingering injuries or near misses – muscle memory goes a long way.
Solutions for Safer Workspaces
Better labeling stands out – not just “irritant,” but real warnings based on honest experience. Regular training and quick refreshers keep facts in people’s heads. Good design separates work and break areas, so lunch doesn’t happen in the shadow of lab glassware. For large users of the acid, local exhaust, spill kits, and real PPE make workplace mistakes less likely. Store it tight, cool, and dry to keep it stable. Reviewing spill protocols every few months, rather than once a year, makes a difference.
Safety with chemicals like 2,3,4-Trifluorobenzoic acid comes down to more than rules— it means acting on the wisdom learned from near-misses and small incidents, and learning from those who came before us.