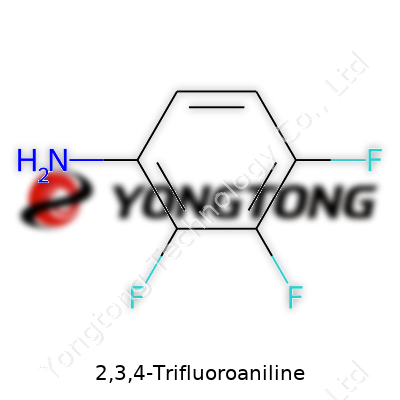
2,3,4-Trifluoroaniline: Insights, Uses, and the Road Ahead
Historical Development
Aromatic amines have a rich legacy rooted in the early growth of organic chemistry. Chemists honed in on anilines to drive forward dye and pharmaceutical industries long before trifluorinated compounds entered the spotlight. As fluorination techniques developed over the 20th century, derivatives like 2,3,4-trifluoroaniline emerged as valuable tools for medicinal and material science. The introduction of fluorine atoms into the aniline ring offered a way to guard molecules against metabolic breakdown and altered their reactivity for synthesis. Now, researchers can access a wide class of trifluoroanilines, applying them far beyond what early chemists imagined.
Product Overview
2,3,4-Trifluoroaniline stands out as a specialty chemical, part of the broader class of aromatic amine fluorides. Its utility reaches across laboratory and industrial landscapes, drawing interest from fine chemical manufacturers, pharmaceutical developers, and agrochemical researchers. Suppliers typically offer this compound in small-to-medium batches, premised on changing research needs and the complexity of its application. Navigating sourcing remains a challenge for some, especially with fluctuations in supply chains or purity requirements, so industry participants keep a close eye on both global markets and lab-scale suppliers.
Physical & Chemical Properties
Here, 2,3,4-trifluoroaniline takes on a clear to pale yellow appearance, usually as a liquid at room temperature. Its chemical formula, C6H4F3N, signals the impact of three fluorine atoms replacing hydrogens at specific positions on the aromatic ring. This substitution shifts physical behavior compared to plain aniline—one can notice lower solubility in water, higher lipophilicity, and differences in boiling and melting points. The molecule’s boiling point ranges around 70-100°C at reduced pressure, while its strong electron-withdrawing groups tune its acidity and reactivity. Adding or subtracting even one fluorine atom changes handling protocols, so knowledge of its exact structure remains crucial in the lab.
Technical Specifications & Labeling
Suppliers label 2,3,4-trifluoroaniline with significant detail, including CAS number, molecular weight, chemical structure, and purity (usually above 98% for research applications). Each batch passes through rigorous quality assessments, measuring water content, residual solvents, and common impurities. Clear hazard labeling reflects both its amine character—which can impart acute toxicity—and the fluorinated functional groups, often flagged under local chemical safety legislation. For transport and storage, guidance focuses on glass or compatible high-density plastic containers, under a dry, inert atmosphere, and away from strong oxidizers or acids.
Preparation Method
Synthetic access routes dictate both cost and practicality. Chemists most frequently employ halogen exchange or selective nucleophilic aromatic substitution, starting from appropriately substituted nitrobenzenes or halogenated anilines. Catalytic hydrogenation of nitro precursors opens the amine functionality, while controlling temperature and reagent ratio secures the right substitution pattern. Anyone who’s spent long hours at the bench can recall the careful tweaking of conditions—each fluorine atom’s introduction changes electronics across the aromatic ring, demanding a deep understanding of reaction kinetics and selectivity. Developing scalable, green, and less hazardous processes is a constant focus for both academic and industrial teams.
Chemical Reactions & Modifications
Chemists exploit the reactivity of 2,3,4-trifluoroaniline on several fronts. The electron-deficient ring alters electrophilic aromatic substitution, slowing certain pathways but opening windows for C-N or C-C bond formation under catalytic conditions. N-acylation gives access to amides for materials chemistry, and diazotization paves the way for functional group interconversions. When forming biaryl connections or coupling reactions, the substituents guide regioselectivity and suppress unwanted side reactions. Modifying the amino group converts the core into a platform for heterocycle construction or further fluorine incorporation—a wellspring for drug discovery campaigns.
Synonyms & Product Names
Researchers may encounter various names across journals and catalogues: 2,3,4-trifluorophenylamine, α,β,γ-trifluoroaniline, or the registry-assigned labels from suppliers. This variety calls for vigilance—mislabeling during procurement can slow down projects or create safety concerns. Using precise nomenclature in record-keeping reduces errors, and referencing CAS 393-87-5 builds consistency when collaborating across borders or disciplines.
Safety & Operational Standards
Handling trifluoroanilines involves measured caution. Direct exposure risks skin and eye irritation, and inhalation can harm respiratory health. Teams make a point to use gloves, lab coats, and goggles, and to steer clear of open flames or faulty extraction systems. In my own laboratory days, robust fume hoods and spill kits proved essential—no shortcut ever justified even the smallest safety lapse. Manufacturers abide by OSHA or REACH guidelines, matching risk profiles with appropriate response plans and regularly updating personnel on best practices. Waste streams flow into dedicated disposal circuits to contain any toxic byproducts or persistent fluorinated residues.
Application Area
The applications of 2,3,4-trifluoroaniline stand out in medicinal chemistry. Fluorinated aromatics resist metabolic degradation in vivo, extending half-lives and modulating activity in drug candidates. Agrochemical developers look for similar properties, crafting pesticides or herbicides where breakdown rates matter for safety and efficacy. Material scientists incorporate fluorinated building blocks to design polymers with improved thermal or chemical resistant properties. Even academic groups leverage its unique reactivity for exploring new synthetic approaches or mechanistic studies. Small-scale users and high-throughput platforms both benefit from its availability, though cost and regulatory paperwork factor heavily into wider adoption.
Research & Development
Interest in new fluorinated amines grows with every leap in medicinal or materials science. For those working in drug discovery, trifluoroanilines provide a scaffold to enhance molecular interactions with proteins or enzymes. Research teams analyze structure-activity relationships, probing how the trifluoro motif changes pharmacological profiles. Process chemists pursue green alternatives to traditional synthetic steps, adjusting solvents, catalysts, and energy input to boost sustainability. Collaboration between manufacturers and end-users often shapes improvements, especially when downstream applications drive tighter purity or regulatory standards.
Toxicity Research
Toxicologists keep a watchful eye on aromatic and fluoroaromatic amines. Animal studies flag cautionary signals: acute exposure can disrupt hepatic or renal function, and certain amines raise mutagenic or carcinogenic potential under specific conditions. Environmental fate also matters—the persistence of fluorinated organics raises questions about long-term accumulation in water sources or soil. Ongoing research looks to clarify degradation pathways and biological impacts. Data gaps persist, so responsible users back up safe handling with targeted studies and updated workplace guidelines.
Future Prospects
Demand for precise molecular modifications shows no slowdown. The trifluorinated aniline group will feature in the next wave of smart therapeutics, advanced materials, and diagnostic tools. Chemists are working to lower the cost of fluorination, aiming to make such scaffolds even more accessible. Regulatory shifts might tighten restrictions, especially around environmental persistence, nudging innovation toward non-persistent or biodegradable alternatives. Advancements in machine learning and reaction informatics will likely streamline new synthetic routes and predict beneficial modification patterns. From my perspective, close partnerships between academia and industry stand to accelerate the safe and purposeful integration of 2,3,4-trifluoroaniline into products that benefit society while respecting safety and environmental responsibilities.
A Closer Look at 2,3,4-Trifluoroaniline’s Structure
2,3,4-Trifluoroaniline draws interest from chemists and material scientists because of its unique arrangement of atoms. Anyone familiar with organic chemistry can picture a benzene ring as the backbone. Aniline tacks an amino group (NH2) onto this six-membered ring. In 2,3,4-trifluoroaniline, three fluorine atoms attach to positions 2, 3, and 4 around the ring, changing how the molecule behaves. These specific spots matter: they control reactivity, shape, and what kind of reactions this molecule can join.
Why Placement on the Benzene Ring Changes Everything
Once you start swapping out hydrogen atoms on a benzene ring for elements like fluorine, the character changes. Each fluorine at position 2, 3, or 4 pulls electron density away from the ring, making certain sites less reactive, and others more suitable for further reaction or binding. In my time working in lab settings, I’ve watched researchers favor these trifluorinated versions for selective synthesis — the placement of each fluorine isn't an accident but a careful chess move. When you're chasing a pharmaceutical target or trying to build specialized polymers, location always rules.
Breaking Down the Structure in Simple Terms
Imagine the benzene ring as a clock. Attach NH2 at the “12 o’clock” spot. Fluorine jumps onto the next three spots — “1 o’clock” (position 2), “2 o’clock” (position 3), and “3 o’clock” (position 4). The rest of the ring sticks with standard hydrogen atoms. This placement gives 2,3,4-trifluoroaniline its name and its special physical properties — from melting point to how it reacts under different lab conditions. Fluorine isn’t just small and light; it packs a punch by drawing electrons and creating new possibilities for chemistry.
Applications and Influence in Research
My own experience meeting chemical engineers and drug development teams shows just how often trifluorinated anilines pop up. They use 2,3,4-trifluoroaniline as a building block, mostly because fluorine’s presence can alter biological activity, make molecules more stable, or change how they interact in a complex system. For instance, a lot of modern drug molecules sport fluorine atoms. The reasons tie back to metabolism — certain enzymes just can’t break down these rings as easily, making medication last longer.
Environmental chemists give trifluorinated aromatic compounds a second look since fluorine offers a unique resistance to breakdown. This can become a double-edged sword when those molecules end up where they shouldn’t. So in the chemical industry, people pay attention to not just how to make these fluorinated compounds, but also the safest ways to dispose of or recycle them. Guidelines from agencies like the EPA now push for best practices around fluorinated products, and I see more conversations about green alternatives or advanced filtration systems to prevent accidental release.
Addressing Health and Environmental Concerns
Making, using, and cleaning up after compounds with fluorine brings responsibility. Testing for toxicity and persistence forms part of any serious research plan. Industry standards increasingly demand transparency about these compounds’ life cycles. Researchers keep pushing forward—using advanced catalysis, designing selective degradation using other chemicals, or inventing better containment during synthesis and transport. The balance between harnessing the power of trifluorinated aromatics like 2,3,4-trifluoroaniline and managing their risks shapes the future of laboratories and manufacturing floors alike.
Rare Molecule, High Demand
2,3,4-Trifluoroaniline doesn’t have a name that rolls off the tongue, but its impact reaches farther than you’d guess. I once walked through a lab while a chemist explained how even the smallest tweaks to chemical structures can change everything about a product. Three fluorine atoms, tucked into an aniline ring, shift reactivity, stability, and toxicity in ways that chemists count on. This chemical often shows up as an essential ingredient, not a finished product. Think of it like yeast in bread: you don’t see it, but nothing rises without it.
Fueling New Pharmaceuticals
Drug research keeps looking for shortcuts to better medicine. Chemists use 2,3,4-Trifluoroaniline as a building block, or “intermediate,” when crafting new drugs. Stick a few fluorine atoms onto an aromatic ring, and suddenly the whole molecule interacts with the body in completely new ways. For example, some antibiotics and anti-inflammatory drugs owe their power to structures built on foundations like this. By tweaking the fluorine placement, researchers control whether a drug lasts longer, works faster, or targets one part of the body more than another.
Adding fluorine often helps drugs resist breakdown from enzymes in the liver. Researchers from the University of California published a paper showing that small changes, like trifluorination, boost the success rate of candidate compounds by making them tougher and more selective in action. This translates to fewer side effects for patients and longer shelf lives for manufacturers.
Chemical Industries Rely on Smart Tools
It’s not just pharmaceuticals. Companies making pesticides and herbicides also pull 2,3,4-Trifluoroaniline into their processes. Many of the latest “selective” agricultural chemicals come from synthesis routes that start with specialty amines like this. By swapping in fluorine, chemists make products toxic to weeds or bugs but less harmful to crops and people.
I spent a season on a commercial vegetable farm, and the difference between old-school herbicides and the new, fluorinated ones was real—plants stayed healthier, and water runoff didn’t smell nearly as harsh. Data from the EPA support these experiences, highlighting reduced environmental persistence and improved breakdown of targeted pesticides built from modern fluorinated scaffolds.
Materials Science: Smaller Parts, Bigger Advances
Technology keeps shrinking the scale for electronics and materials. 2,3,4-Trifluoroaniline steps in when engineers make advanced polymers used for electronics, membranes, and coatings. Its fluorine atoms add resistance to heat and chemicals, so products last through lots of wear and rough environments. The result? Circuit boards, chemical sensors, and water-treatment membranes with longer working lives.
Seeing these updates from manufacturers in Japan and Germany, it’s clear how global supply depends on tight control during synthesis. Purity and consistency matter, because flaws lead to failure in microchips and sensors. Research from major electronics suppliers confirms that trifluoroaniline-based monomers anchor structures that don’t break down easily under thermal or electrical stress.
Responsible Chemistry: What’s Next?
The strengths of 2,3,4-Trifluoroaniline come with challenges. Making and using fluorinated chemicals demands tight oversight. Health professionals want clearer toxicity data, and environmental experts look at persistent chemical buildup. Solutions might come from greener chemistry—using renewable resources for synthesis or finding safer breakdown routes after use.
Tighter global safety standards can guide this work. Manufacturers now publish more about their sourcing and waste management. By combining innovation with responsibility, the industries that rely on specialty amines like 2,3,4-Trifluoroaniline can help control risks and deliver products that make a difference.
What Makes 2,3,4-Trifluoroaniline Tricky?
2,3,4-Trifluoroaniline sounds like just another chemical, but the risks are real. It’s part of the aniline family, and folks who’ve worked with these know skin and lungs don’t get along with even small spills or whiffs. This one adds extra bite with those three fluorine atoms, ramping up the volatility and toxicity. If you’ve ever gotten even a whiff of aniline, then you know how harsh it can be—throat stings, eyes water, sometimes headaches roll in fast. This isn’t one of those “skip the gloves” compounds.
Preparation Makes All the Difference
Work starts long before you ever uncap the bottle. Every chemist owes a lot to their prep game: safety data sheets on the desk, chemical fume hood checked and running, and the right gloves chosen—something like nitrile or neoprene, since latex breaks down too fast with aromatic compounds. Eye protection, never negotiable. The lab coat might seem symbolic to outsiders, but splash just once and you’ll never forget that layer. Closed-toe shoes don’t just keep you in dress code; they protect from spills that love to find ankles.
Ventilation Is King
Trying to measure out 2,3,4-Trifluoroaniline in open air takes things in the wrong direction. The fumes do more than stink—they can set off asthma, cause dizziness, and after repeated hits, lead to serious health problems. A sturdy fume hood does more than just keep air moving. It acts like a shield, letting you keep focus instead of worrying about a pounding headache or itchy throat mid-weighing.
Spills and Splashes Happen—Then What?
Even with the tightest technique, every lab veteran can recall a spill, sometimes at the worst time. Knowing the drill matters as much as good pipetting skills. Small spills call for absorbent pads and neutralizing powders, all handled with gloved hands. For bigger messes, evacuate the area before trying to clean up—self-reliance sounds noble until you’re the one needing rescue. Eyes or skin hit? Straight to the eyewash or safety shower, no hesitation, fifteen minutes minimum. That always feels long, but short-cutting it brings trouble down the line.
Waste Plans Prevent Bigger Hazards
Tossing 2,3,4-Trifluoroaniline down the drain sends the problem downstream. Labeling waste, keeping it in a sealed, chemical-resistant container, and logging it for hazardous material pickup doesn’t just follow rules. It protects others, preserves air and water, and keeps surprise audits from turning into real emergencies. Burning off these compounds is never home chemistry; ask the pros who handle chemical disposal.
Training and Teamwork Over Lone Wolf Moves
Getting proper training isn’t some bureaucratic hoop. Every hour spent on safe handling beats dealing with accidents. Real lab culture looks out for each other—no one scoffs if you pause to check a safety sheet or double up on gloves. If anything feels off during handling—strange scent, mild symptoms—step away and bring it up. Silence in the lab only helps the hazard.
Stay Ready, Stay Safe
Working with 2,3,4-Trifluoroaniline won’t ever be routine for smart chemists. Preparation, gear, and attentiveness make the difference between an ordinary workday and disaster. Trust the basics, lean on your team, and never stop respecting what these chemicals can do.
Digging Into the Numbers
Chemistry doesn’t always spark excitement for most people, but the details hidden in chemicals open doors to countless scientific and industrial innovations. Take 2,3,4-Trifluoroaniline, for example—a compound used in research and synthesis. Its molecular weight clocks in at 149.1 grams per mole, calculated from its structure: six carbons, four hydrogens, one nitrogen, and three fluorines. Knowing this number sounds straightforward. Yet, for folks working in labs or developing new materials, this little value saves money, prevents mistakes, and helps advance important projects.
Why This Number Matters in Practice
Chemical reactions often act like recipes. The right amount of one ingredient changes the outcome just as much as it does in the kitchen. In my student years, one miscalculation led to a failed batch of product, all because someone rounded a molecular weight the wrong way. Those grams add up fast—and the wrong mass of starting material can throw off research, lower a yield, or even waste expensive reagents. The number for 2,3,4-Trifluoroaniline isn’t just a trivia answer. It gives researchers the power to run reactions accurately, order raw materials, and keep costs in check.
Fluorine’s Heavy Hand
Three fluorine atoms boost this compound’s mass more than you might guess. Compare aniline’s molecular weight to its trifluorinated cousin. Regular aniline weighs in around 93.1 grams per mole. Slap those three fluorines on, the mass jumps by 56 units. This fact shapes safety sheets, waste calculations, and shipping considerations. Take it from anyone who has hauled or weighed laboratory chemicals: a heavier molecule can change how much hazard waste a company produces over the course of a year. Regulators often look at these data points for compliance and safety rules. Having the correct molecular weight, down to the decimal, knocks out guesswork and keeps paperwork straight.
Developing New Molecules: Real-Life Impact
The detail in a single number ties into much larger questions. Chemists use 2,3,4-Trifluoroaniline for making pharmaceuticals, agrochemicals, or advanced polymers. Developing a new drug begins with building blocks like this. The molecular weight points directly to dosing, solubility, and even how the body might process a medicine. I’ve watched colleagues explain to non-scientists why a gram of one compound does something completely different from a gram of another, just because of details like molecular weight. Effective communication and clear math break down those barriers between science and other fields—finance, manufacturing, even regulatory affairs.
Getting the Math Right and Keeping Access Transparent
Digital references and standardized databases, like PubChem or Sigma-Aldrich, keep accurate data at everyone’s fingertips now. But mistakes still creep in. I’ve seen labs use old data tables or scribbled notes from a predecessor. Problems follow—misordered chemicals, quality control headaches, and even retractions in research. Regular auditing of records and staff training helps. Limited budgets sometimes make that difficult, so open-access information and peer-reviewed resources show their value again and again.
Looking At Better Solutions
Reliable information paves the way for progress. Investing in up-to-date databases, training staff to double-check numbers, and promoting scientific literacy outside the lab all reinforce better outcomes. Simple tools, like molecular calculators online and clearly labeled bottles in storage rooms, cut back on small yet costly errors. Town-hall style meetings—where researchers, administrators, and safety teams review recent mistakes—can offer real, actionable fixes that go deeper than scolding or paperwork. Getting the molecular weight of 2,3,4-Trifluoroaniline right is more than a calculation. It’s a foundation for safety, accuracy, and innovation.
Understanding the Real Risks
2,3,4-Trifluoroaniline isn’t your everyday chemical. Its uses pop up in labs and specialty industries, but plenty of people—myself included—don’t realize just how important proper storage becomes until the stakes become personal. Working in an organic synthesis lab, I’ve seen what happens when chemicals get handled carelessly: a minor spill can send everyone into emergency cleanup mode, and sometimes it’s a near miss with something much more serious.
The chemical's structure hints at the need for caution. It contains three fluorine atoms attached to an aniline base. That means volatility, possible toxicity, and a real potential for environmental harm if it gets loose. Not every bottle sitting on a shelf gets treated this way, but this compound earns the respect.
Physical and Chemical Hazards
2,3,4-Trifluoroaniline isn’t flammable in the way that, say, ethanol is. Still, it’s classified as hazardous—breathing in vapors or getting it on your skin sets you up for health problems. The presence of amines and fluorinated groups pushes exposure concerns even further. Safety datasheets flag risks of inhalation, eye and skin irritation, and environmental toxicity.
From personal experience, I’ve seen where poor labeling or lax storage turns a well-run operation into chaos overnight. Mix-ups or container leaks cost not just time, but sometimes jobs or even long-term health.
Building A Responsible Storage System
Proper storage keeps everyone safer and protects the environment. I learned early on that cool, dry, well-ventilated spots go a long way in keeping volatile substances from misbehaving. With this compound, plastic or glass containers with tight seals work best—metal sometimes reacts with anilines or fluorinated chemicals, so sticking to the right materials is essential. I always watch for incompatibility, since acids, strong bases, and oxidizers shouldn’t share the same storage location.
Clear, consistent labeling matters. Everything needs to be tagged with the correct chemical name, concentration, and hazard warnings. Even in a rush, skipping this step leads to confusion or dangerous assumptions. Every lab I’ve been part of keeps a central log, often digital, listing each chemical’s storage spot and its expected shelf life.
The Role of Guidelines and Legal Requirements
Most workplaces and academic labs get guidance from OSHA or local agencies—these rules set a baseline, but it often helps to go further. I remember one university that standardizes secondary containment trays for every hazardous liquid. One spill stays contained, so it doesn’t turn into a full lab shutdown.
Ventilation can’t be an afterthought. Regular air exchange in storage rooms and fume hoods goes beyond ticking boxes for inspectors—it prevents vapors from building up to dangerous levels. I’ve witnessed the difference after switching to an updated system: fewer emergency alarms, no headaches, and a safer work environment for newcomers and veterans alike.
Looking Forward
Training fresh faces makes a big difference. New team members who see why procedures matter rarely cut corners. Refresher trainings—whether it’s yearly or after an incident—help everyone stay sharp. Institutions that provide regular updates on chemical storage not only stay compliant but also reduce accidents and near misses.
The bottom line for storing 2,3,4-Trifluoroaniline (or any specialty chemical) boils down to respect—for the hazards, for the people you work with, and for the broader community. Putting the effort into safe storage isn’t about bureaucracy. It’s about real lives and a safer world.