2,3,4,5,6-Pentafluorobenzoyl Chloride: A Deep-Dive Commentary
Historical Development
Chemists looking back at the story of 2,3,4,5,6-pentafluorobenzoyl chloride see a compound deeply connected to the history of aromatic compounds and halogenation chemistry. Fluorinated aromatics, once considered synthetic curiosities, have become critical building blocks for much of the high-performance chemistry that defines modern science. In the decades following the Second World War, techniques for introducing multiple fluorine atoms onto benzene rings opened the door to dozens of new molecules, chief among them being perfluorinated acyl chlorides. Researchers realized early on—often by trial, error, and small-scale success—that the high electronegativity of fluorine could fundamentally change the reactivity and stability of these aromatic cores, and so 2,3,4,5,6-pentafluorobenzoyl chloride emerged from this wave of innovation. Through the years, this tightly-packed fluoroarene became a favorite starting material in the toolbox of synthetic organic chemists.
Product Overview
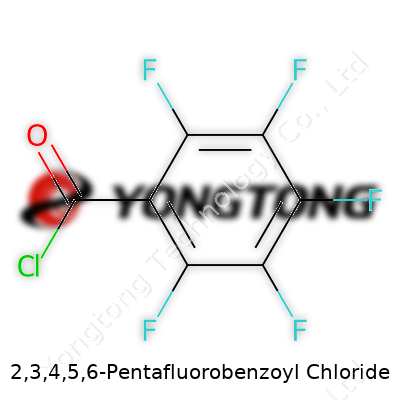
2,3,4,5,6-Pentafluorobenzoyl chloride stands out for both its structural simplicity and chemical potency. The molecule features a benzene ring with each hydrogen swapped for a fluorine, all except the one position that carries an acyl chloride group. To most chemists, it's known for being pungent, irritating, and highly reactive—just the way a good acyl chloride should be, except with the added force of perfluorination. It packs punch as a coupling agent and an activator in syntheses where more mundane acyl chlorides often fall short. Sometimes it's called pentafluorobenzoic acid chloride or just ‘PFBCl’ in lab shorthand. These names show up on bottles, research papers, and safety data sheets, but the molecule itself doesn’t change: it’s a highly electron-deficient acyl chloride that carves a special niche in modern synthetic routes.
Physical & Chemical Properties
This colorless to pale yellow liquid brings some unique physical traits: boiling at roughly 80-82°C under reduced pressure, and giving off dense fumes. Its strong odor catches attention right away—a reminder to handle with proper ventilation. As a member of the perfluorinated aromatics club, it resists both acids and bases in many cases, but the acyl chloride group still invites hydrolysis and nucleophilic attack. The molecular formula is C7ClF5O, and it weighs in at about 230.5 g/mol on the balance. Density runs high, often about 1.58 g/cm³, showing how much mass those five fluorines really add. For anyone handling these chemicals daily, there’s more than academic interest in these numbers—they give you clues on storage, transfer, and reaction setup, whether in a university lab or a small-scale specialty chemical operation. Talking to other researchers, I’ve noticed that the unmistakable traits of this reagent tend to linger in memory (and in the fume hood) long after the reaction wraps up.
Technical Specifications & Labeling
Most suppliers label 2,3,4,5,6-pentafluorobenzoyl chloride by its chemical name, formula, and purity—often aiming for 97% or better. The appearance of the bottle, the distinctive warning phrases, and the unique UN number (usually 3265 for corrosive liquid, acidic) reinforce a culture of care and diligence. The packing usually guards against light, moisture, and air, with PTFE-lined caps proving invaluable in my own experience. In technical terms, buyers expect full disclosure on impurities—such as pentafluorobenzoic acid or unreacted pentafluorobenzene—from reputable suppliers. The correct GHS labeling (flame, exclamation mark, and corrosion pictograms) signals the real-world hazards to anyone working with the substance, underscoring the respect it commands even before breaking the seal.
Preparation Method
Making this acyl chloride at scale usually means starting with pentafluorobenzoic acid, which itself takes some skill to make by direct fluorination or from pentachlorobenzene. The transformation to the chloride relies on powerful chlorinating agents—thionyl chloride, oxalyl chloride, or phosphorus pentachloride all work, though thionyl remains the workhorse. Heating the acid with thionyl chloride drives off sulfur dioxide and hydrogen chloride, leaving behind the pure pentafluorobenzoyl chloride. On a practical note, I’ve found that controlling the temperature and working under dry conditions makes all the difference in yield and product quality. This is no place for shortcuts; loose technique saps purity and can leave behind corrosive by-products, complicating both downstream chemistry and worker safety.
Chemical Reactions & Modifications
2,3,4,5,6-Pentafluorobenzoyl chloride reacts with a wide array of nucleophiles to form esters, amides, and anhydrides that carry the perfluorinated aromatic group. That makes it handy for introducing electron-poor aryl groups into organic molecules—a trick valued highly in materials and medicinal chemistry. On a personal project, I once leveraged this itself as an activating group to streamline a peptide bond formation, discovering that its electron-withdrawing power really speeds things along. In other cases, it’s been used to modify alcohols, refining their reactivity and solubility for analytical purposes. Further, this reagent can undergo Friedel-Crafts-type acylations only with stubbornly unreactive aromatics, avoiding overreaction or side-product headaches. Each modification taps into the molecule’s dual personalities: the aggressiveness of acyl chlorides, and the subtle but powerful influence of fully-fluorinated aromatics.
Synonyms & Product Names
Anywhere you find modern chemistry—the pharmaceutical pipeline, advanced materials labs, or specialty synthesis—this compound goes by a bunch of names. Pentafluorobenzoic acid chloride, perfluorobenzoyl chloride, PFBCl, and even the unwieldy 'benzoyl chloride pentafluoro' all indicate the same substance on bottles and invoices. Looking through catalogs, I’ve noticed that some suppliers use CAS number 434-64-0 as the main identifier, cutting through the jumble of trade names and in-house designations. This short list of synonyms matters for procurement, compliance, literature searches, and communication across teams and borders.
Safety & Operational Standards
In the lab, pentafluorobenzoyl chloride calls for more than just gloves and goggles. Its acyl chloride moiety means it reacts with water, alcohols, and amines—including those found in skin and mucous membranes—to release highly irritating hydrogen chloride fumes. I’ve seen colleagues underestimate the need for double containment or reliable fume extraction, only to find themselves scrambling when white fumes curl up from the reaction flask. Corrosivity, toxicity, and volatility all come into play, making a written risk assessment and up-to-date SDS not just bureaucratic steps, but real shields against harm. Waste disposal calls for careful neutralization and segregation, something I learned firsthand after an exothermic mix-up in a shared waste drum. Institutions with robust chemical hygiene plans expect regular audits of storage and procedures for reagents like this, aiming to catch risks before they turn to incidents. These safety steps filter into every stage, from delivery to synthesis to clean-up—a series of best practices built on both lessons learned and regulatory requirements.
Application Area
Fluorinated acyl chlorides have become vital in pushing forward technology on all fronts—from pharmaceuticals and agrochemicals to advanced polymers and analytical standards. Chemists reach for pentafluorobenzoyl chloride when they need to make molecules that resist breakdown, repel water, or disrupt biological systems for a targeted effect. It features in derivatization protocols for GC-MS, where its unique electron-withdrawing profile shifts analyte volatility and detection profiles in a predictable, useful way. Medicinal chemists use it to fine-tune the metabolic stability or receptor specificity of a drug candidate, sometimes just by swapping out a methyl for a pentafluorophenyl group. Polymers built from these units show up in coatings, membranes, and specialty films demanding chemical resistance far beyond the usual materials. My own experience has shown how one well-chosen fluorinated fragment can transform an entire synthesis route, saving steps or unlocking new reactivity—justifying both the expense and the strict handling rules.
Research & Development
Research teams leveraging pentafluorobenzoyl chloride are driving ahead in materials science, surface engineering, and drug discovery. Much of the current work explores novel coupling partners, aiming to turn this reactive intermediate into new architectures—multi-arm dendrimers, non-stick coatings, and even fluorescent markers for bioimaging. Analytical groups study its derivatization chemistry to reach ever-lower detection limits for trace analysis, often tweaking the reaction conditions to reduce thermal degradation or side-product formation. I’ve read recent papers where researchers use it to protect fragile functional groups during complex syntheses, then gently remove the pentafluorobenzoyl tag under mild conditions. Each advance depends not just on the chemistry, but on the skills and creativity of the people involved. Even more, those chasing greener chemistry keep working on reducing the need for hazardous chlorinated reagents or chlorinating agents, trying to solve both scientific and environmental puzzles at once.
Toxicity Research
The safety profile of pentafluorobenzoyl chloride centers on its corrosivity and acute inhalation toxicity, both from the acyl chloride and the dense, choking hydrogen chloride that it can release. In toxicology studies, exposure in animals triggers inflammation, tissue damage, and respiratory distress; direct contact can produce severe chemical burns. The fluorinated aromatic core itself raises questions—while most researchers consider perfluoroaromatics less likely to bioaccumulate than their alkyl cousins, the by-products of incomplete reactions or combustion open up further risks. Long-term research continues into how trace exposures shape both human health and environmental spread, with regulatory agencies pushing for tighter controls. These efforts blend exposure monitoring, cellular assays, and real-world accident case reports, building up the knowledge base that shapes modern handling protocols. In my own time working with this chemical, I’ve seen researchers take pains to avoid not just acute exposures, but also chronic, invisible risks—a responsibility that weighs on every experienced chemist.
Future Prospects
The story of 2,3,4,5,6-pentafluorobenzoyl chloride is far from over. Its future lies in med-chem design, smart polymers, highly selective derivatization agents, and as a backbone for molecular electronics. Demand for ever-more-robust materials and targeted pharmaceuticals drives ongoing interest, so researchers worldwide work on expanding production, improving safety, and lowering environmental footprint. Advances in green chemistry may, before long, bring less harsh routes to its synthesis, or even bio-based alternatives for key precursor chemicals. As the regulatory landscape tightens, the pressure grows for manufacturing and research operations to invest in upgraded containment, monitoring, and cleaner synthesis. With every new publication or industrial breakthrough, chemists refine both the science and the stewardship of this valuable yet demanding compound.
A Gritty Real-World Chemical
Digging through the shelves of a research lab or paging through a material safety data sheet, a bottle labeled 2,3,4,5,6-Pentafluorobenzoyl Chloride jumps out — not just for the mouthful of a name, but for the way it fits into the world of high-precision chemistry. This is not an everyday chemical found under the kitchen sink. Here’s what stands out to someone like me with a foot in both the science and practical world: This compound acts as a bridge to help scientists shape molecules in ways that push technology, agriculture, and even medicine forward.
Sharp Tool for Making Connections
In organic chemistry, forming connections between molecules is both an art and a puzzle. 2,3,4,5,6-Pentafluorobenzoyl Chloride brings a special edge. I’ve used compounds like it to activate carboxylic acids — basically revving up their reactivity. That extra push lets chemists link up all sorts of building blocks — amino acids, alcohol groups, and more — laying the groundwork for peptides, pharmaceuticals, polymers, or even just a better HPLC detector tag. You add this stuff to a flask, and, suddenly, you’re building more than you could before.
Why Go Fluorinated?
Stacking five fluorine atoms on the benzene ring doesn’t just look flashy; it changes how the molecule behaves. The electron-hungry fluorines draw power away from the rest of the system, cranking up reactivity. I have seen how this can mean cleaner, more predictable reactions. Sometimes, the target molecule wobbles close to impossible with other chemicals, but the fluorinated benzoyl chloride pulls it together. If you want a reaction to finish fast and with fewer annoying byproducts, you reach for something like this. In analytical labs, attaching one of these benzoyl groups makes small and hard-to-see molecules much easier to detect. Suddenly, your analysis isn’t squinting at a foggy result — the peaks pop right up.
Moving the Needle in Research
Sometimes, just a dash of the right chemical opens doors. In my experience, switching from a standard benzoyl chloride to a pentafluorinated version flips yields from disappointing to satisfying. Researchers making new antibiotics, crop-protecting agents, or surface coatings often run into stubborn roadblocks — low reactivity, side reactions, or poor selectivity. A tough, electron-thirsty reagent like this pushes reactions to finish cleanly, sometimes unlocking whole new areas of discovery. For those building new molecules to study disease mechanisms or environmental toxins, pentafluorobenzoyl chloride turns what could be a months-long slog into a focused sprint.
Responsibility and Safety
Using this compound means respecting some unforgiving properties. Pentafluorobenzoyl chloride gives off strong fumes and burns skin on contact — anyone in the lab knows not to take shortcuts here. Full gloves, face shields, working fume hood, no exceptions. I’ve had my own run-ins with similar reagents, and there’s no such thing as too careful. The fact that its power in synthesis matches its hazards reminds me that chemistry always walks a line between creation and risk.
Beyond the Bottle
A chemical like 2,3,4,5,6-pentafluorobenzoyl chloride rarely makes the news, but it hides behind countless advances. Though it doesn’t grab headlines, each step forward in drug development, agricultural chemistry, or materials design can trace a faint fingerprint back to specialized reagents just like this one. Bridging tricky connections, sharpening detection, and pushing the boundaries — all from a small bottle waiting on a crowded lab shelf.
Looking After This Tricky Chemical
2,3,4,5,6-Pentafluorobenzoyl chloride brings its own quirks to the workbench, just like many reactive acyl chlorides. My own experience in chemistry labs has taught me respect for substances that react so fast and so hard with the world around them. You let your guard down, and moisture in the air will start picking apart those sensitive molecules, sometimes even before you get the lid back on.
Shutting Out Moisture and Light
Hydrolysis remains the biggest threat. Pentafluorobenzoyl chloride readily breaks down into pentafluorobenzoic acid and hydrochloric acid whenever water creeps in. I’ve opened jars after a few careless days—white mist vapor, that vinegar-sharp tang, and liquid turning yellow. This is both a health risk and ruin for your chemical’s purity. Keeping reagent bottles sealed up tight matters more than just about anything else. Labs trust glass bottles with teflon-lined caps for good reason—they really do keep moisture at bay. Silica gel packets near your bottles help keep ambient humidity low in cabinets where these chemicals wait.
A Cool, Dark Place—Not Just a Cliché
Temperature can tip the balance between stable storage and slow breakdown. Heat speeds up unwanted reactions, including hydrolysis and self-polymerization. Pentafluorobenzoyl chloride handles normal room temperature better than many acid chlorides, but I’ve seen sluggish bottle caps fail on a summer day, letting vapors escape. Keeping chemicals like this below 25°C, preferably in a dry refrigerator or designated cold chemical room, just makes sense. If your lab’s fridge runs steady at about 4-8°C, you’re in the safe zone. Don’t put it beside lunch containers, either—chemical fridges really ought to be chemical-only.
Ventilation, Containment and Safety—Here’s Why
Even capped tight, small leaks add up. Vapors from pentafluorobenzoyl chloride can cause serious irritation to eyes and respiratory tracks. More than one chemist learns the hard way about the faint but persistent sneaky smell. Specialty flammables cabinets protect from accidental contact and help contain leaks. These cabinets provide added fire resistance, so if a bottle falls or a fire breaks out, you’re at less risk of noxious clouds spreading. Well-ventilated spaces matter more than you think—chemical vapors shouldn’t hang around, even at low concentrations.
Labeling and Regular Checks Prevent Surprises
Old labels peel, notes fade, and before you know it, mystery bottles lurk on dusty shelves. Proper labeling—chemical name, concentration, date received, and hazard warnings—saves time and headaches. Set a habit to check every few months for color shifts, cloudiness, or crystals around the cap. These subtle signs can mean compromised purity, leaky seals, or worse. Disposing of degraded reagent promptly protects everyone in the facility.
Learning from Mishaps
In my own work with fluorinated benzoyl chemicals, small lapses led to big inconveniences: ruined batches, time wasted, and disposal costs multiplying. No win comes from keeping hazardous chemicals past their shelf life. Reach for what you’ll use soon and order smaller containers as projects demand them. Training others on proper handling, real storage concerns, and watching out for early warning signs keeps the whole team safer. Respect for chemistry means respect for the people working with these chemicals every day.
Personal Experiences in the Lab
Few lab chemicals command respect like 2,3,4,5,6-Pentafluorobenzoyl chloride. Years ago, I knocked an opened bottle over during a late-night synthesis, and I can still remember the burning sensation in my throat. Hay fever-like sneezing didn’t go away for hours. Regret followed carelessness, and I learned a lesson many chemists do: gloves, goggles, and fume hoods aren’t optional. Safety basics keep accidents from becoming disasters.
Why This Stuff Demands Caution
2,3,4,5,6-Pentafluorobenzoyl chloride reacts sharply with moisture, and even sweat counts. Skin contact leads to corrosion. Air exposure releases hydrogen chloride gas, which stings the eyes and nose. Acute respiratory damage sometimes follows brief exposure. Folks in the lab world talk about “respecting the hood,” and with a material like this, it’s more of a commandment than a suggestion.
This compound doesn’t just threaten the person holding the pipette. Leaky glassware lets fumes drift across the bench. It pays to think beyond your own project and consider the whole shared environment.
Best Practices Backed by Fact
Good containment is more than laboratory theater. According to published safety data, undiluted pentafluorobenzoyl chloride produces corrosive vapors at room temperature. Gloves rated for strong acids—think nitrile or neoprene, not latex—block splashes. Safety goggles close the gaps around your eyes. A fitted lab coat acts as the first line of defense, and nothing beats a properly ventilated fume hood. Working out on the bench tempts fate.
If a spill happens, absorbent pads soaked in sodium bicarbonate neutralize splatters. Going straight for water splashes acid in your face, so it’s much safer to use neutralizers followed by careful rinsing under running water if any touches skin. Seek medical attention even after minor exposure—delays often make it worse.
Labeling and Storage: Where Accidents Hide
Poorly labeled vials wind up in the wrong hands. Accidents show that, in crowded fridges, similar-looking bottles wind up switched. Storing this chemical in its original container with tight seals and clear hazard symbols beats improvisation. Keeping it away from water sources or basic reagents avoids unwanted reactions.
Inventory logs don’t just please regulators, they help others figure out what’s in a container after labels wear off. Chemical safety officers always check logs before clearing out an old cabinet. That habit saves money—and sometimes much more.
Training and Emergency Preparedness
Nothing makes me cringe more than a new lab member handed a bottle without guidance. Even students catch on fast when shown emergency showers and eye-wash stations before the first experiment. Regular drills sharpen everyone’s response times. Some institutions have taken to posting QR codes linking to up-to-date digital safety data sheets, making real safety information always accessible.
It’s tempting to cut corners on a busy day, but safety standards exist because sharp lessons left scars. Respecting the risks protects careers, health, and sometimes lives. In the end, diligence wins every time.
Chemical Reality in the Lab
It’s no surprise that chemists often confront the quirks of chemicals that sound intimidating or complex. I remember the first time I dealt with anything “pentafluorinated." A hasty assumption had me reaching for the water to clean up, hoping it'd dissolve easily. I learned quickly: pentafluorinated chemicals like 2,3,4,5,6-pentafluorobenzoyl chloride don’t usually play nice with water.
Why This Compound Matters
Universities and pharmaceutical labs use 2,3,4,5,6-pentafluorobenzoyl chloride in organic synthesis, especially for making amides and esters. Its power comes from the five fluorine atoms, which pull on electrons and make it highly reactive toward other chemicals. This feature turns it into a go-to reagent in creating specialty molecules—you might even spot its name in methods for peptide synthesis or surface modification.
Water Solubility Reality Check
Let’s get down to the main question: does this compound dissolve in water? Straight to the point—almost not at all. Look at its structure: a benzene ring covered with fluorines, capped with a reactive acyl chloride group. Fluorine atoms hate water because they build up a strong, non-polar character that doesn't bond with water molecules. On top of that, the acyl chloride part reacts with water rather than mixes with it. You get a violent splash, a burst of heat, and soon you’ll smell acid in the air—hydrochloric acid to be precise.
Textbooks confirm this; solubility charts and safety data sheets mark 2,3,4,5,6-pentafluorobenzoyl chloride as “reactive with water” and not “soluble.” During my graduate years, I kept plenty of gloves and goggles handy—spilling even a few drops in water meant a short dash to the fume hood.
Scientific Facts Support the Practice
The numbers back it up. Acyl chlorides react with water through hydrolysis, forming benzoic acids and hydrochloric acid gas. Fluorinated benzoyl chlorides make this even messier. These compounds barely make it into solution before breaking down. Lessons from chemical safety courses and published literature drive home the point—water won’t help you dissolve or dilute this compound; you’ll only get a hazardous reaction.
Why This Matters for Researchers and Industry
Ignoring this fact is risky, especially outside of well-ventilated labs. People sometimes look for quick solutions, but with this compound, that choice invites danger and ruined reactions. Common solvents like dichloromethane and acetonitrile or dry ether are much safer for handling, transport, and reaction cleanup.
Safe disposal also calls for special containers and trained personnel. Facilities must keep water away from any spill kit dealing with acyl chlorides, especially those amped up by fluorines. Even at scale, manufacturing outfits using this material double-check protocols to protect staff and the environment.
Real Solutions for Safer Handling
The safest approach: double-check material safety data sheets, educate every team member, and never skip personal protective equipment. Outsourcing disposal to a certified handler helps prevent water contact disasters. Anyone handling these chemicals should get hands-on instruction in a real lab before solo work with this reagent.
Knowing 2,3,4,5,6-pentafluorobenzoyl chloride and water don’t mix keeps chemists and staff safe. If you’re tempted to reach for the tap, read up, gear up, and swap in a compatible organic solvent. Every smart, safe move keeps labs humming and discovery on track.
Getting the Details: Formula and Weight
Diving straight into the chemistry, 2,3,4,5,6-Pentafluorobenzoyl chloride carries the molecular formula C7ClF5O. No jargon, just the facts. Each molecule contains seven carbon atoms, five fluorine atoms, one chlorine, and one oxygen. Its molecular weight clocks in at 230.52 g/mol. These numbers offer a clear window into the molecule's personality. Anyone working with custom syntheses or specialty reagents in the lab will want to keep those details handy, not just for record keeping but for handling, dosing, and predicting reactivity. Jumping into work without these figures often leads to errors in stoichiometry and can waste valuable time or even compromise safety.
Why Formula and Weight Matter in Real Labs
Here’s the thing: chemistry isn’t just about formulas written on paper or computer screens. Behind each number is a real-life implication, stretching from the way you order your compounds to the way you plan out a reaction and measure each reactant. That molecular weight, 230.52, tells you how much to weigh out for a precise mole. You’ll want every decimal point right, especially if you’re scaling up from a successful test batch to a liter-sized reaction. A missed calculation can turn a good day in the lab into a mess, both on the bench and in the records. The formula also offers clues about reactivity — all those highly electronegative fluorines and the stubborn chlorine demand caution, protective gear, and good ventilation.
Practical Impact: Beyond the Numbers
Experience in organic synthesis teaches you to respect even the tiniest details. Pentafluorobenzoyl chloride is not just another acid chloride. The five fluorines ramp up its electron withdrawal, making the molecule both more reactive and trickier to handle compared to vanilla benzoyl chloride. Reactions using it tend to move faster, sometimes with greater selectivity, especially in forming amides or esters where standard benzoyl chloride falters. Its size and electronegativity influence solubility, volatility, and the way side reactions creep into what should be a clean conversion.
This isn’t just theory. In pharmaceutical development, those fluorines and that chlorine often show up in drug candidates because they bring more than just mass — they affect metabolic stability, bioavailability, and how a drug interacts with proteins. Researchers use those unique features intentionally, often swinging the odds in favor of a more effective or more stable drug.
Facts Backed by Evidence
Reliable sources like PubChem and peer-reviewed journals place this compound’s formula and mass in alignment with the ones given here. Synthetic chemists lean on this kind of validated data since regulatory filings, analytical results, and safe handling practices all depend on accuracy. Risking outdated or unverified figures can hold up a project or risk a failed quality audit.
Challenges and Solutions in the Modern Lab
To keep things running smoothly, invest in digital inventory platforms that integrate chemical properties. These let you pull up every relevant property instantly, minimizing the risk of error and saving time digging through catalogs. Invest in good training: knowing why those numbers matter sticks better than rote memorization. Stock your chemical storage with clear labels and easy-to-read hazard warnings, especially for reactive chlorides like this one. Encourage open discussions when you or your colleagues run into issues – shared experience is often the best safeguard against repeated mistakes. The chemistry world moves fast, but the foundation remains buried in the basics.