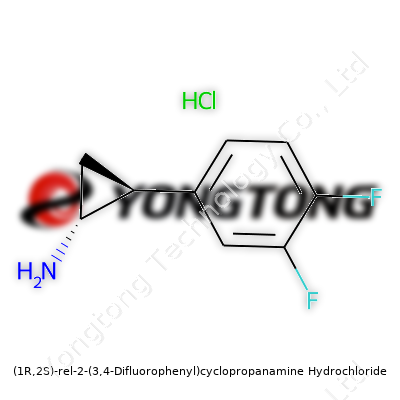
(1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine Hydrochloride: More Than Just a Chemical Ingredient
Historical Development
Every chemical tells a story, and few narratives take more unexpected turns than that of cyclopropanamines. The journey toward (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride traces back to early organofluorine research in the latter half of the twentieth century. Scientists working with cyclopropane derivatives watched the rise of selective serotonin reuptake inhibitors and related nervous system agents, often seeing value in introducing fluorine atoms to change biological activity. Over time, the addition of difluoro groups to aromatic rings became a standard way to manipulate lipophilicity and metabolic resistance in new drugs. The process of finding the right chiral geometry led to the development of this compound, balancing synthetic complexity with desirable pharmacological profiles. Major milestones shifted from basic synthesis improvements to scalable routes, with patent literature revealing an increasing focus on purity, safety, and clinical promise.
Product Overview
(1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride lands squarely at the intersection of medicinal chemistry and process optimization. Researchers find value in its clean pharmacophore and defined stereochemistry. This salt form solves solubility and handling issues encountered with free amines. Manufacturers typically offer it as a white to off-white crystalline powder, immediately recognizable to those who’ve spent time weighing out research materials in gloveboxes and fume hoods. Consistent product quality remains essential, especially for researchers scaling up reactions for animal studies or clinical trials.
Physical & Chemical Properties
Aromatics bearing two fluorine atoms and a strained cyclopropane ring make for a molecule with unusual stability and reactivity. (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride usually melts between 170 - 180°C, though the precise point depends on subtle variations in crystalline habit or solvent inclusion. It dissolves easily in water and polar solvents, but stays generally stable under ambient conditions when stored away from light and moisture. Having handled similar salts in the past, one quickly learns that avoiding even brief exposure to humidity protects both yield and purity. Its molecular formula (C9H11F2N·HCl) shapes everything from its density to its boiling point. This profile often points to broad utility in medicinal chemistry, where predictable behavior supports demanding analyses.
Technical Specifications & Labeling
Every container leaving a reputable manufacturer carries more than a name and weight. Proper labeling includes batch numbers, purity specifications—routinely 98 percent or higher—and validated analytical data. High-performance liquid chromatography (HPLC) traces become almost as familiar as the product’s own faintly acidic scent. Laboratories appreciate clear expiration dates and safe handling reminders, especially those managing high-throughput synthesis or preclinical assays. Getting to this level of reliability often takes years of process development, troubleshooting, and regulatory navigation.
Preparation Method
Making (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride requires a strategic mind and clean benches. The most common route relies on asymmetric cyclopropanation, beginning with substituted cinnamic acid esters or related intermediates. Transition-metal catalysts, often rhodium-based, control the chirality, guiding the synthesis to yield the desired (1R,2S) geometry. Following ring construction, chemists introduce the amine group through well-practiced reductive amination or similar techniques. The hydrochloride salt forms in situ by treating the amine with hydrogen chloride, often in an alcoholic solvent. Each step demands careful purification, sometimes involving column chromatography or crystallization, to meet stringent pharmaceutical standards.
Chemical Reactions & Modifications
Chemical curiosity rarely stops with the parent molecule. In research labs, chemists rapidly test ways to tweak the cyclopropanamine motif. Halogen exchange, N-alkylation, and formation of the carbamate derivatives enable a vast landscape of analogs. Those who work in medicinal chemistry know that swapping even one fluoro atom can shift the entire pharmacokinetics profile. Amid all these possibilities, the base compound’s robust aromatic ring and strained aliphatic group lend themselves well to further functionalization without compromising integrity. Care must be taken with strong acids or bases, as the cyclopropane ring does not forgive uncontrolled conditions—splitting the ring can eliminate the compound’s value in a single misstep.
Synonyms & Product Names
Chemists and commercial suppliers juggle a handful of names for this compound. “2-(3,4-difluorophenyl)cyclopropanamine hydrochloride” regularly joins research orders. CAS numbers stay close at hand, helping avoid expensive mix-ups in ordering and inventory management. In global markets, slight spelling variations or translation quirks appear, but the core identity remains stable among those who rely on this molecule for advanced synthesis projects. Most catalogs append “(1R,2S)-rel-” to signal the stereochemistry that keeps research outcomes consistent.
Safety & Operational Standards
Safe handling protocols matter. Lab workers wear gloves, lab coats, and safety glasses when measuring and transferring this hydrochloride salt. Any spills land in the solid waste bin after containment with absorbent pads. Ventilation keeps fine dust out of lungs. Material Safety Data Sheets provide clear-cut hazard categories and recommend adequate ventilation, eye protection, and prompt spill response. Even without strong acute toxicity, respect for the unknown guides careful disposal and storage. Observing rigorous safety standards reduces the long-term health risks faced by workers who handle hundreds of chemicals every month.
Application Area
This compound travels far beyond the bench. Researchers chasing central nervous system targets use cyclopropanamine derivatives as part of lead compound libraries. Fluorination increases blood-brain barrier permeability, sparking new trials in neuropsychological studies. Teams synthesizing agricultural or analytical reagents also draw on its stable backbone for making diverse compounds. Some even see potential in developing radiolabeled analogs for imaging or diagnostic technologies. Access to pure, well-characterized starting material can determine the difference between a promising drug candidate and months wasted reworking the chemistry.
Research & Development
In both academic and industrial settings, (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride draws attention thanks to its chiral structure and metabolic resilience. Development teams invest in new synthetic routes that minimize waste and avoid hazardous reagents. Early research highlights its unique pharmacological properties, with many groups leveraging it as a cornerstone for selective receptor modulator programs. Having worked through plenty of chiral separations, I recognize the appeal of a single-enantiomer product that avoids racemization headaches downstream. Scale-up demands innovation—reaction intensification, solvent recycling, and in-line analytics all play roles in delivering larger quantities without sacrificing quality.
Toxicity Research
Though not broadly flagged as hazardous in small amounts, this hydrochloride salt still falls under rigorous toxicological scrutiny. Safety testing in preclinical research looks at acute and chronic oral, dermal, and inhalation effects. Select animal models reveal organ-specific interactions or metabolic byproducts that differ from non-fluorinated analogs. Regulatory bodies want to see full documentation before approving any compound for advanced studies or large-scale production. Material that once looked benign under a microscope sometimes exhibits subtle neurological, hepatic, or renal effects—highlighting the value of thorough, ongoing research and transparent data sharing across organizations.
Future Prospects
Looking ahead, (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine hydrochloride seems poised to remain a vital research tool. Advances in asymmetric catalysis promise faster, cleaner access to the chiral amine class. Drug discovery teams keep searching for new neurological applications, while agrochemical researchers develop better pest control strategies based on cyclopropane frameworks. The intersection of analytical chemistry and machine learning hints at more predictive design and safety screening, trimming development costs and speeding regulatory review. For anyone invested in the practical side of chemistry, growth depends on robust supply chains, collaboration between laboratories and manufacturers, and a solid commitment to transparency about environmental and long-term health impacts.
What’s Important About This Compound
You may notice this chemical’s name sounds like a tongue twister, but inside many research labs, it sparks more than a passing interest. (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine Hydrochloride finds its strongest footing in the drug discovery field, especially among folks working on neuroactive compounds and psychiatric medications. What stands out immediately is the cyclopropane ring bolted to a difluorophenyl group—structures that pop up in several pharmaceuticals. Chemists pay close attention to such tweaks because small changes can swing a molecule’s activity or safety profile in surprisingly big ways.
Why This Compound Shows Up In Research
Researchers working to design better antidepressants or therapies for central nervous system disorders reach for molecules like this cyclopropanamine. Structurally, it echoes the backbone of some established drug families called monoamine oxidase inhibitors or serotonin-norepinephrine modulators. My time in the lab, shadowing a medicinal chemist, showed me just how fast things move. A minor chemical tweak—like adding those two fluorines to the phenyl ring—can help dial in potency, reduce metabolic breakdown, or lessen nasty side effects. These adjustments are why compounds like this one aren’t only logged into chemical libraries but also screened routinely for biological activity.
The use of hydrochloride salt forms, such as this compound, isn’t an academic afterthought. Those salts make it easier to handle the compound, boost its stability, and sometimes help with absorption in the body. This impacts everything from storage on a laboratory shelf to potential use as a drug down the line. In the past, our lab sessions often stalled with oily, unstable amines—getting a crystalline, more manageable hydrochloride version made all the difference.
Safety And Ethical Testing
Handling new psychoactive candidates can't just rest on good science. The standards for developing anything that touches the brain require rigorous safety testing and transparent data. Academic and biotech teams carefully weigh toxicity, long-term effects, and the ethics of animal or cell-based studies. There’s hardly a shortcut here: regulatory groups call for detailed reports before these candidates move even an inch toward human testing. My old mentor used to say, “Nothing leaves this bench until it's been looked over ten ways to Sunday.” That standard still matters, especially with molecules that show promise for impacting neural circuits.
Where Paths Lead Next
Efforts to create smarter psychiatric drugs press on. Industry and academic players run parallel tracks, tweaking base structures like that found in (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine Hydrochloride. Some modify the cyclopropane ring for receptor selectivity. Others explore dosage forms that sidestep the liver’s metabolism, aiming to get cleaner results and fewer interactions. These choices respond to real frustration with side effects, resistance, or patient dropoff in traditional medication approaches.
With ongoing advances in machine learning and screening technology, candidates like this one don’t stay obscure for long. Automated platforms sort through thousands of derivatives, turning up hits that move to animal trials far faster than in past decades. The future might hold new medicines born from structures recognizable in this compound, reworked for next-generation needs.
Gaps and Possible Solutions
A big challenge remains in translating positive lab findings to actual benefits for people living with depression or neurological conditions. Predicting how small modifications will behave in a real brain, with all its messy chemistry, pushes scientists to improve their models. Lab workers must lean on both cutting-edge analytics and old-fashioned skepticism. Broadening datasets to include more genetically diverse populations—and increasing transparency in reporting—would help build the trust that’s sometimes missing in psychiatric drug development. Sharing results openly and honestly moves everyone closer to medicines that don’t just look good on paper but deliver lasting, safe relief in the real world.
Why Purity Matters in Everyday Operations
Purity isn't just a fine print detail tucked away on a certificate. In any job where chemicals come into play—be it labs, manufacturing, or even cleaning—it's the first thing I check before anything else. Once, during a routine analysis, I watched a project fall apart just because we overlooked a 0.5% impurity. That tiny margin introduced unpredictable reactions, wasted hours, and pushed delivery dates back by a full week. So, it's not just a matter of ticking a box. Impurities, even in trace amounts, can change the outcome, affect safety, increase costs, and ruin credibility.
The Building Blocks: Specifications Set Expectations
Chemical specification sheets read like instruction manuals for safety and reliability. They usually define properties like chemical formula, molecular weight, melting point, and moisture content, alongside purity. These numbers stabilize decision-making for purchasing, storage, and disposal. Once, I received a drum without proper documentation—the team didn't touch it until we got the full breakdown from the supplier. If something’s going into your process, trust only grows with transparency. A batch with unknowns or vague specs doesn't just raise eyebrows; it risks health, lawsuits, and lost trust.
Trust Built on Data, Not Hype
Recent years have seen high-profile recalls in sectors ranging from food to pharmaceuticals, and the root cause nearly always traces back to a gap in specification or verification. According to the FDA, over 60% of drug recalls link to impurity-related problems. Contaminants don’t always advertise themselves; sometimes they slip through because the vendor only provided an assay value without a deeper breakdown. Anyone relying solely on numbers like “99% pure” is asking for trouble—what makes up the other 1%? That remainder isn’t always harmless filler. I’ve learned to dig in and ask for full impurity profiles, residual solvents, and heavy metal content before I sign off.
Questions Worth Asking for Safer Operations
We can’t improve what we don’t measure. It's worth grilling suppliers: How did you test purity? Which standards do you follow—USP, ACS, ISO? Has the product passed microbiological checks, or could bacteria wreck our batch? I push for external lab certificates wherever living up to regulatory audit matters and check if updates match recent batch numbers. A slip-up in documentation, or a mismatch between the lot and the paperwork, says something got missed in quality assurance.
Learning from Mistakes and Setting Solutions
Years ago, we had a close call with a solvent containing unexpected stabilizers. Only after running a GC-MS screening did the puzzle pieces click. That taught me to lobby for a two-step supply process: primary screening from the vendor, plus independent lab verification. Many companies skip this due to costs, but a single bad batch can wipe out far more in lost time and liability than routine checks would ever cost.
The Right Way Forward
Building better habits matters most. Ask for data before moving forward with a new batch, cross-check against authoritative methods, and cultivate direct relationships with suppliers who understand why you ask “What’s really in this product?” early and often. Regulatory bodies aren’t satisfied with vague assurances and neither should we. In the end, attention to purity and specification isn't just for the lab coat crowd—it’s a shared responsibility that keeps our systems running safely, efficiently, and with the trust our clients expect.
Why Storage Matters for Specialty Chemicals
Anyone who’s spent time working with research chemicals knows no two molecules behave the same. Some powders cake after meeting humid air. Others shift color with a temperature spike. (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine Hydrochloride isn’t much different. Its hydrochloride form means it attracts water more than many organics, pulling moisture from air and changing consistency or potency. Whether you measure milligrams for drug development or catalog compounds for a screening library, nobody wants a sample that’s turned clumpy or lost its punch.
Temperature: Your First Line of Defense
I’ve seen researchers try room temp, but those little fluctuations add up. Ideal practice keeps this cyclopropanamine at 2–8°C, using a reliable refrigerator or, if space allows, a cold storage walk-in. Fluctuating above 8°C can shift its stability, and hitting freezer levels (below -20°C) can introduce condensation during thaw cycles. Lab freezers also risk frost build-up, which rarely helps small-molecule integrity. Cool—but not frozen—wins the long game, making a chill in the fridge the safest bet.
The Enemy: Moisture and Air
Opening a container and finding a cake of formerly free-flowing powder frustrates even the most patient chemist. I remember opening a batch that had wicked in so much moisture it wouldn’t dissolve in standard solvents—wasted time and money. Drierite or fresh silica gel packets tuck inside storage bins and absorb ambient moisture. Instead of screw caps alone, I prefer double-sealed containers—wrap vial caps and then seal them in a foil-lined pouch or a sturdy zip-top bag. Air exposure triggers oxidation and can nudge the hydrochloride to drop its proton, so minimize opening containers more than necessary.
Light: Friend or Foe?
White fluorescent bulbs won’t fry this molecule right away, but over time, light can prompt unwanted side reactions. Keep vials in amber glass or opaque plastic. Cardboard secondary containment works, but a true lab sees fewer mishaps with hard-sided containers—too many times I watched a box get jostled off a bench and spill open. Simple fixes like storing away from open windows and harsh LED desk lamps keep your chemicals from yellowing or degrading before their time.
Labeling Matters More Than You Think
Half the hassle in chemical storage comes from hunting through faded or incomplete labels. I label with permanent marker and wrap clear packing tape over every code, date, and hazard symbol. Include batch number, received date, and initials. If a compound comes undated or half-labeled from a supplier, re-label it before you shelve it. If someone else needs it months later, clear identification means less confusion and safer retrieval.
Disposal and Accidental Release
Once I had a container break, spilling a chunk of this compound onto the lab bench. If this happens, scoop solids into a sealable waste jar. Clean the surface with soapy water. Don’t touch bare skin—use gloves, change out lab wipes, and air out the area. Waste gets logged with hazardous chemical protocols, matching your institution's requirements.
Building a Storage Culture
I’ve worked in labs where one person’s loose habits turned a clean sample collection into a mess inside of a year. Set clear storage guidelines for everyone. Post temperature, humidity, and labeling rules inside chemical storage areas. Review shelf stability logs every three months, and rotate stock as often as possible to avoid letting anything expire or degrade unseen on the back of a forgotten shelf.
Each tiny vial of (1R,2S)-rel-2-(3,4-Difluorophenyl)cyclopropanamine Hydrochloride costs money and time, not to mention the risk tied to a ruined experiment. Everyone in the lab keeps quality high by holding the line on careful, detailed storage habits—because the next breakthrough could start with a single, uncontaminated gram.
Everyday Encounters with Chemical Compounds
You don’t have to work in a lab to cross paths with interesting chemical compounds. Many jobs, from janitorial work to healthcare, involve some degree of contact with substances that sound intimidating on a label. I remember my time in a hospital storeroom—one moment you’re unpacking saline, the next you’re face-to-face with hydrogen peroxide far stronger than that brown bottle at home. Every time, I double-checked what I was touching, because any slip-up could mean a mess, a ruined uniform, or something far worse.
Why Safety Data Sheets Save More Than Just Time
The Safety Data Sheet (SDS) is the first thing I reach for before handling anything unfamiliar. This document tells you exactly what a substance is capable of—whether it irritates skin, needs ventilation, or shouldn’t touch water. Some compounds behave harmless in storage, but go sideways with friction or a little heat. The SDS lays out basic concerns, like whether it sparks up easily or turns toxic with air. You only need one experience fumbling through an emergency to realize guessing is a poor plan, especially with chemicals that can change the health of anyone around.
Personal Protective Equipment Isn’t Just for Show
Early in my career, I treated goggles like a chore until liquid splashed alarmingly close to my eyes. Instant regret taught me: real protection stays on, even for “quick” tasks. Safety goggles, gloves, aprons—they all feel inconvenient until they stop a spill from burning through your skin or ruining your vision. Nitrile gloves resist most substances better than the classic latex, and eye shields rated for chemical duty don’t fog up as fast as cheap ones. Nobody wants extra laundry or a trip to the clinic, so gearing up right away becomes part of muscle memory.
Storing Chemicals: It’s Not Just About Neat Shelves
I’ve seen more than one storeroom jammed with bottles on whichever shelf fit. That’s a recipe for disaster. Strong acids and bases start breaking down shelves and each other if stored together. I learned to check labels twice before putting anything away, making sure oxidizers, flammables, and water-reactives live in their own zones. Keeping these apart limits exposure during leaks, earthquakes, or accidents nobody sees coming. No one ever wants to be mopping something up, only to have it react explosively with what’s already there.
Training and Emergency Prep: No Room for Shortcuts
Short-staffed shifts lead people to cut corners with safety, but experience proves that half the hassle of emergencies comes from not knowing plans beforehand. Fire blankets, eyewash stations, evacuation routes—familiarity with these keeps trouble small. Regular practice drills matter. Even if some coworkers roll their eyes, the first time you use an eyewash or need to guide someone out of a foggy storeroom, the whole team benefits from quick, steady action.
Practical Solutions Build Lasting Habits
Relying on good labels, storing everything correctly, and wearing PPE every time builds trust and safety in any workplace. Routine checkups on supplies, fresh training, and open conversation about close calls all improve everyone’s odds of leaving work unharmed. Chemicals don’t forgive shortcuts, and a few moments reading the right information give you more control every day. That’s something everyone can carry from storerooms and labs into their own homes.
People Want Straightforward Information
Everyone shops online or requests quotes for work. The real frustration comes from websites that bury the most basic info: the price and whether the product is in stock. Imagine browsing for an air purifier, industrial part, or even a popular gadget. You click, read through pages of “features” and wade through jargon, but still can’t figure out if you can actually buy it, or how much you’d have to pay. Most of us just close the tab and move on. According to a study by the Baymard Institute, nearly 70% of online shoppers abandon their carts. One big reason: uncertainty in pricing or stock.
Clear Communication Builds Trust
Out of curiosity, I sometimes reach out to suppliers for chemicals, tools, or electronics. The best ones send transparent quotes quickly. Sometimes they include a full breakdown of costs—unit price, bulk discounts, shipping fees. Right away, I get a sense of what I’m dealing with. The sellers who respond promptly earn my trust. Once I bought 20 circuit sensors from a small business in Wisconsin. Their website displayed real-time stock counts, so I didn’t worry if my order would be delayed. That transparency kept me coming back, even if the price was just a bit higher.
Vague Answers Push Customers Away
It doesn’t only frustrate buyers; sellers miss out too. A Gartner survey found that 89% of businesses compete mostly on customer experience. Hiding pricing behind “contact us” forms rarely works out for the shopper. Shoppers get suspicious. They worry about hidden fees or the hassle of calling for a quote. Worse, they might end up with a salesperson who pushes extras instead of answering a simple question. Most people click away before sending an email for a basic product. Clear updates on pricing and whether something is in stock improve both sales and reputation.
Why Accuracy and Expertise Matter
Wrong details about price or stock level make the process a headache for both sides. Product managers who keep info up-to-date show they know their inventory and market. If something is backordered, say so. Everyone prefers a “Ships in 3 weeks” note over being strung along with empty promises. I once needed safety equipment for work, only for a high-profile retailer to admit they’d shipped the wrong item after a week of silence. Accurate details save time and stress.
How to Fix It: Make the Information Obvious
Expertise shows when sellers keep product listings fresh. Top companies track their inventory with warehouse systems and connect those results to their website or customer portal. For pricing, set clear baseline prices and spell out discounts for larger orders. Sellers should avoid generic “Request a quote” buttons unless the item is truly special-order. Low-tech businesses can post an updated PDF price sheet or at least a catalog with prices. Someone on staff needs to check this info at least weekly.
Sellers who respect buyers’ time post accurate info up front. That transparency shows real-world experience and earns repeat customers. In an age where trust and expertise matter more than ever, a quick answer to “Can I buy this, and for how much?” can set a business apart.