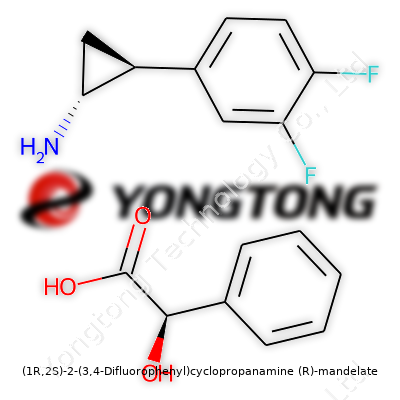
(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate: A Close Look
Historical Development
Years ago, chemists hunting for new structural backbones kept an eye on cyclopropane amines for their rigidity and unique reactivity. The addition of a 3,4-difluorophenyl group onto a cyclopropanamine ring started drawing attention in the late twentieth century when pharmaceutical research ramped up the hunt for novel antidepressants and central nervous system agents. Adding the mandelate salt, particularly the (R)-stereoisomer, gave researchers a way to manage chirality, solubility, and stability—problems that had been plaguing earlier synthetic efforts. The evolution from simple amines to this complex, highly specified salt reflects the growing understanding of how small shifts in structure change everything from biological activity to physical stability.
Product Overview
This compound stands ready as a crystalline salt, often off-white, that has found purpose both as a chemical intermediate and as a closer-to-final building block in pharma synthesis. Each batch often ships with a certificate of analysis, detailing not just the rotamer ratio or optical rotation, but the entire story of that particular run—impurities, water content, enantiomeric excess, residual solvents—everything that a regulatory auditor or experienced bench chemist wants to know. Global manufacturers have invested in reliable supply chains to ensure that researchers and developers never run short, especially since this salt sometimes lands as a key precursor to active drug substances.
Physical & Chemical Properties
Dry, granular, and with a faint, often bitter smell, (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine (R)-mandelate dissolves easily in methanol and ethanol, moderately in water, but tends to clump in organic solvents with low polarity. Its melting point hovers in the range of 145°C to 150°C, making it stable enough to survive most purification steps, but not so sturdy that recrystallization becomes a chore. The compound holds its own under ambient humidity, as long as storage avoids long exposure to open air. Subtle but important, the optical rotation signals its enantiomeric purity—something every medicinal chemist pays attention to, because it means the difference between a compound that heals and one that harms.
Technical Specifications & Labeling
Manufacturers print labels that do more than protect legal liability; they bring the bench chemist up to speed immediately. Besides traditional identifiers like purity percentage (98% and higher is standard for drug research), labels show lot numbers, production dates, and often QR codes linking to digital documentation. These records include IR, NMR, HPLC chromatograms, and even the synthetic route, allowing a thorough audit trail for both quality and traceability. The presence of fluoride atoms and sensitive cyclopropane rings means shelf-life and hazardous categorization also show up clearly—details ensuring safe handling along every research and manufacturing stage.
Preparation Method
Laboratories usually start with 3,4-difluorobenzaldehyde, tapping either a Simmons–Smith cyclopropanation or an asymmetric reductive amination to build the (1R,2S) core. Resolving racemic mixtures typically involves chiral acids, with (R)-mandelic acid performing double duty as both a resolution agent and a counterion for improved stability. Once the amine forms, careful pH control guides it into a stable salt, which then undergoes filtration, drying, and recrystallization. Each synthetic run brings the challenge of contamination—fluorous byproducts, over-cyclopropanation, or the formation of undesired stereoisomers—that has to be monitored and removed by chromatography and meticulous purification.
Chemical Reactions & Modifications
The functional groups here offer plenty of places for further chemical tinkering. Medicinal chemists have exploited this molecule’s primary amine for reductive alkylation, while the difluorophenyl ring becomes a target for Suzuki and Buchwald–Hartwig couplings. Modifications on the cyclopropane—though more difficult—promise unique changes in pharmacology, though that often comes at the cost of increased synthetic complexity. Careful hydrogenation can open the cyclopropane, giving researchers a jump-off point for totally new analogs. Early research also showed modest but real sensitivity to acid–base extremes, nudging chemists to keep reactions in buffered conditions or short time scales during scale-up.
Synonyms & Product Names
Depending on the supplier, you might see this compound marketed as “(1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine (R)-mandelic acid salt” or its more compact handle “DFCPA mandelate”. Some catalogs use internal alphanumeric tags, while others stick close to the systematic nomenclature, sometimes swapping in a “hydrochloride” or “free base” descriptor if the salt form changes. Whenever researchers order or reference it in publications, full clarity on stereochemistry and counterion is a must—leaving no room for mix-ups that can derail a month’s worth of experiments.
Safety & Operational Standards
Working with this compound in the lab means much more than wearing gloves and goggles. Benzene derivatives often pose chronic toxicity risks, and fluorinated aromatics, though less volatile than some, still warrant working under a hood. The cyclopropane ring, if handled improperly during downstream processing, can occasionally release reactive fragments that demand robust ventilation or containment. Material safety data sheets from major suppliers detail not just acute exposure effects (irritation, CNS depression) but long-term environmental persistence for fluoride-containing debris. Waste management becomes a central theme, ensuring residual solids and wash solvents don’t find their way into general trash or the drain. Sustained training on these standards shields research teams from occupational hazards and keeps organizations on the right side of environmental regulations.
Application Area
The heartland for this salt sits squarely in drug discovery. Its enantioselectivity and rigid structure offer tight fits in protein binding pockets, making it a frequent guest in SAR programs for antidepressants, antipsychotics, and even experimental oncology drugs. Biotech startup teams see it as a springboard to custom analogs, layering on functionalities to chase efficacy or minimize toxicity. Academic groups have explored it as a reference point in stereoselective catalysis studies or chemical biology projects where chirality drives molecular recognition. Its presence in patent filings hints at a wider circle of influence—especially in preclinical studies where tweaking the sidechain or aromatic ring means one more chance at a breakthrough compound.
Research & Development
Over the last decade, research groups picked apart not only the binding properties but also the metabolic fate of this salt. Stable isotope labeling allows tracing every atom through human liver microsome assays and in vivo models, while microfluidic purification tools trim down prep time. Collaborative efforts across academia and industry have steadily refined synthetic routes, shaving off waste, boosting yields, and moving toward greener chemistry. Investment in process analytical technology, especially real-time NMR and IR flow monitoring, helps catch batch-to-batch inconsistencies long before they threaten a regulatory filing. The compound's chiral center remains a magnet for computational chemists exploring ligand–protein docking, optimizing lead compounds moored to the same scaffold.
Toxicity Research
Preclinical labs don’t take chances with new fluorinated structures. Rodent studies have flagged some CNS activity at moderate doses, mostly transient and resolving without intervention. Long-term dosing in animal models usually checks for any hint of organ toxicity or immune response that might scuttle later clinical development. In vitro genotoxicity screens have rarely shown worrying signals, though researchers keep their protocols robust in case of unexpected findings. Metabolite profiling has documented that the cyclopropane core stubbornly resists oxidative breakdown, and the difluorophenyl moiety exits mostly as glucuronide or sulfate conjugates—good news for downstream safety. Still, flags remain about possible accumulation if dosing schedules ramp up or if formulations stretch far from their original intent.
Future Prospects
Looking down the road, the urgency in pharma for highly selective tools makes this class of compounds even more valuable. Generative chemistry powered by AI points to endless analogs spun from this backbone, chasing better binding, longer half-life, or fewer side effects. Manufacturing groups keep nudging process waste lower, aiming for sustainable production to satisfy environmental audits. Regulatory pushes on chiral purity, documentation, and post-market safety nitpick every property, but this relentless scrutiny only sharpens commercial and academic outcomes. Collaborative networks linking chemists, material scientists, toxicologists, and process engineers promise faster routes from bench to bedside, especially as new diseases demand creative problem-solving backed by solid molecular design. The future of these fluorinated cyclopropane salts might not be obvious to every consumer, but in the engine rooms of global research, they’re shaping the next wave of therapeutic breakthroughs.
Why Purity Matters in Precision Chemistry
In pharmaceutical and research chemistry, clean compounds drive results. (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate stands out as a piece of a bigger puzzle in drug development. Purity isn't just a number slapped on a label—it shapes safety and reliability. For researchers, a pure batch means predictable reactions, fewer surprises, and confidence during scale-up.
Digging into Real Purity Numbers
Most suppliers selling this compound cite purity numbers at or above 98%, sometimes even guaranteeing 99% or higher. Analytical certificates typically back this up, highlighting results from HPLC (High Performance Liquid Chromatography) or NMR (Nuclear Magnetic Resonance). Having worked in a lab where every tenth of a percent mattered, I've seen the fallout of skipping purity checks: inconsistent biological activity, noisy spectra, wasted time running down faulty results.
Pharmaceutical standards run tight. The U.S. Pharmacopeia often demands upwards of 99% purity for active pharmaceutical ingredients. Compounds falling below that bar bring risks—unwanted side reactions, unreliable dosing, off-target effects. Fluorinated compounds like this one, with a cyclopropane ring and specific chirality, appear in antidepressant and antipsychotic research. Minor impurities can tangle up the interpretation of an experiment or, worse, skew preclinical results.
How Purity Gets Verified
Methods like HPLC, NMR, and mass spectrometry unmask anything extra hiding in the vial. In my experience, purity testing doesn't just mean scanning for a clean chromatogram—it also tracks enantiomeric excess, since one isomer may carry all the intended biological action while its mirror image muddles the data. Labs sometimes miss out on verifying both chemical and optical purity; the R/S configuration of this amine isn't decorative, it's decisive.
For clients who demand it, some suppliers run chiral HPLC to guarantee enantiopurity. A batch that strays from the target loses its edge. Whenever I've ordered samples out of curiosity from different sources, even a “99% pure” label couldn’t save a project if enantiomeric purity wasn’t up near that number too. The difference between 98% and 99% seems small until you run cell assays or animal studies and the inconsistency shows.
How the Industry Can Do Better
Purity problems plague even the biggest labs. Supply chains break down, subtle contaminants creep in, tracking and transparency slip. Working toward routine batch testing and full documentation can close the gap. I’ve seen more teams request raw data alongside certificates. Some push for open-access batch history—chromatograms and full NMR stacks. Direct contact between end users and chemists helps. Questions get answered faster, and minor blips don’t spiral into major setbacks.
Any chemist who’s spent a late night troubleshooting odd results knows how draining it gets to fix purity issues after the fact. Better upfront testing, not just trust in a CAS number, saves everyone headaches. As standards grow stronger, reliable sourcing and sharp communication keep good science on track. A trustworthy supplier isn’t just about numbers—it’s about giving you enough detail to trust the science in your hands.
Safe Storage: Lessons from the Lab and Real Life
I’ve spent years working with sensitive chemicals, and the nuances of storage conditions shape both the safety and the quality of your investment. (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate is no different. This compound isn’t just another bottle on the shelf; it’s a carefully crafted piece of chemistry that wants respect. Keeping it in good condition preserves its usefulness, avoids waste, and shows care for the tools that scientists and manufacturers put their trust in.
Keep It Cool, Keep It Dry
Every time I open a chemical catalog or step into a supply room, the words “cool, dry place” ring out loud. This combination acts as a shield against most of the things that destroy fine compounds. Temperature swings can cause changes in purity and make the substance less reliable. Moisture creeping into bottles eats away at shelf life and can start unwanted reactions.
I store delicate amines at temperatures below 8°C whenever possible, usually in a dedicated refrigerator. Avoid household fridges packed with food—cross-contamination isn’t something to gamble with. Specialized lab fridges have alarms and internal logs tracking door openings and temperature bumps, so you can spot trouble before an accident happens.
Avoid Light and Keep Sealed Tight
Exposure to light breaks down many fluorinated phenyl compounds over time. Clear glass doesn’t block that, so I reach for amber vials or cover ordinary glass with a protective wrap. A tight seal is critical; air exposure triggers oxidation, which messes with amines in subtle ways you might not spot until it’s too late. Most labs use screw-cap bottles with PTFE liners because even a tiny leak can spell big losses.
Why Documentation and Training Matter
Chemical management goes beyond the right fridge. In every well-run lab I’ve seen, a log sheet rides alongside every specialty container, recording each time it’s opened or aliquoted. This isn’t just red tape. It helps catch patterns—maybe someone left the door open too long or a bottle was handled too roughly. Training helps everyone respect the material and know which gloves, goggles, and fume hood protocols to follow.
Know the Warning Signs
I watch for signs of trouble: color changes, cloudiness, or strange smells. These can mean breakdown is under way. Regular inspection stops questionable material from ending up in a reaction flask, saving hours or days later.
Disposal and Spills
Spilled or spoiled (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine isn’t just a headache for productivity. Unchecked waste causes hazards for staff and the environment. I’ve seen labs with dedicated waste bins for fluorinated amines and regular reminders from supervisors about the right disposal companies to call. Prevention beats emergency cleanup every time.
Moving Toward Best Practice
Storing specialty amines like this one asks for precision, teamwork, and respect for science. The right approach keeps people safe, budgets in check, and important discoveries rolling forward. Cutting corners often creates bigger problems down the road. Careful storage stands as one of the most straightforward investments you can make in quality research and safety.
Why People Keep Asking About COAs
Walk into any supplement shop, CBD store, or even a hardware supply company, and someone eventually pipes up about a COA. Some will tell you they’ll only buy if the seller shows them the paperwork. I remember my first brush with a COA question, years back, after ordering what I thought was a pure vitamin C powder online. Turned out the lab results were a mystery, and I ended up with a bag of orange-tinted dust that tasted like floor cleaner. The lesson was clear: don’t trust the label—see the proof.
Certificates of Analysis Put Promises to the Test
Sellers tend to promise high standards. "Pure, lab-tested, safe," they claim. The COA shows if they’re telling the truth. It’s a report from a lab (ideally unaffiliated with whoever’s selling the stuff) that breaks down what’s actually in the package. For vitamins, you might see levels of ascorbic acid. If you want CBD oil, the COA will show THC levels and check for nasty pesticides.
Who Actually Asks for a COA?
College students buying plant extracts may shrug off paperwork, but parents buying omega-3s for their kids or people trying to manage a medical condition usually demand more than a sticker and a slogan. It’s a habit worth adopting. Real-life stories show why. Not long ago, independent labs found products on popular shopping sites packed with heavy metals or active ingredients way above safe levels. Only a COA, dated and signed, gives clues about what’s on offer.
Not All COAs Are Created Equal
Some companies toss together a flashy PDF with barely-legible graphs and call it good. Make sure the COA lists the lab’s name, the sample batch number, test date, and numbers for key ingredients. Look at the format; a proper COA gives raw data, not marketing lingo. Testing should cover purity and screen for unwanted extras — not just what the seller wants to brag about.
Facts and Recommendations from Experts
Consumer Reports found that over half of U.S. adults worry about contamination in wellness products. This isn’t paranoia—multiple studies, including FDA recalls, have revealed serious safety gaps. Experts recommend asking for a COA with every purchase, especially for items taken by mouth or used on the skin. The COA reduces the risk of taking something harmful or simply ineffective.
How Retailers and Buyers Can Do Better
Retailers should treat each COA as a mandatory part of the sales pitch. Make them easy to find, not something you have to email and hope for a response. Products without clear certification get left on the shelf. For consumers, get in the habit of glancing over COAs, even if the brand seems reputable. It helps to trust your gut—if a seller dodges the question or the paperwork looks fishy, look somewhere else.
Transparency Matters More Than Ever
COAs help honest brands stand out and save buyers from nasty surprises. People want real proof, not just buzzwords. If you care about what goes into your body or your home, asking "Is a COA available for this product?" isn’t being picky. It’s common sense.
Digging Into the Details
Chemists and researchers working with (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate know molecular weight isn’t just a number on a spreadsheet. It tells you about the substance’s fundamental nature, how it might behave in a reaction, and what doses or concentrations actually mean in the real world. The molecular weight of this compound — 323.31 g/mol — comes from tallying the atomic masses of every atom in the formula. Each carbon, hydrogen, nitrogen, oxygen, and fluorine counts. It’s a labor of arithmetic that I’ve repeated more times than I care to count back in the lab.
Precision Drives Progress
Getting an exact molecular weight matters to more than just chemists. In drug development, exact dosages depend on molecular weight. Measuring out 100 milligrams of active ingredient won’t mean much unless the team has nailed down what those 100 milligrams represent on the molecular level. Analytical labs trust this measurement when confirming compound purity through mass spectrometry or HPLC. One deviation in the calculated weight throws off results, leading to questions about the compound’s identity.
Early in my own industry work, I learned the hard way that skipping these details opens the door to a world of problems. Imagine ordering a specialty chemical for a month-long set of experiments, only to find the molecular weight printed on the label is off due to a supplier error. Every calculation, every dose, every interpretation of bioactivity would suddenly be up in the air. That lesson left a mark. I double-checked, even triple-checked these values before trusting any supplier or even my own spreadsheet.
The Link to Purity and Safety
Drug safety connects strongly to molecular calculations. If a pharma company formulates pills or solutions with the wrong molecular weight in hand, actual dosages can spiral out of range. That can mean patients get too little (no benefit) or far too much (toxic side effects). Diagnostic measurements, bioassays, and stability studies all depend on precise molecular weights. Regulatory agencies audit these figures closely, tying compliance and patient trust to accuracy at this scale.
Fluorinated ring systems — like the 3,4-difluorophenyl part in this molecule — add a wrinkle to calculations and outcomes. Fluorine atoms aren’t just numbers; their presence affects solubility, metabolic breakdown, and detection in analytical equipment. Every atom counts in clinical planning and in chemical manufacturing. An overlooked detail here can mean wasted resources or, worse, patient risk.
Building Trust With Transparency
Good science always leans into transparency. Reporting molecular weights, compound structures, and calculation methods empowers others to reproduce, question, or build on any result. I’ve sat in enough meetings where a well-detailed method let colleagues troubleshoot a snag or catch a data error. Every good report I’ve written has included not just the numbers, but the path to them.
Textbook calculations use standard atomic weights: Carbon at 12.01, Hydrogen at 1.01, Nitrogen at 14.01, Oxygen at 16.00, Fluorine at 19.00. Adding up each atom’s contribution for (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate gets you to that precise 323.31 g/mol.
Practical Steps Forward
Laboratory teams can head off confusion by always referencing reputable databases and double-checking supplier data sheets before buying acids, amines, or other specialty reagents. Open communication about numbers and methods stops error chains before they snowball. Years in the field have shown me the value of collective vigilance — catching a typo or miscalculation early saves time, money, and sometimes careers.
The details and the discipline of calculation don’t just keep experiments running — they keep people safe, products pure, and research honest.
The Responsibility that Comes with Laboratory Chemicals
Science pushes boundaries, opening doors to innovation and new medicines. Compounds like (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (R)-mandelate fuel research that can change lives, but a vial in the wrong hands—or down the wrong drain—turns a tool into a threat. Working in a synthetic chemistry lab for years makes it clear: a misstep with even a small sample can have lasting impact on health and the environment.
Personal Safety Comes First
Gloves, goggles, and lab coats must stay on—not as a ritual, but as necessity. Direct skin contact with chemicals like these often leads to rashes, burns, or allergic reactions. Fume hoods aren’t a luxury; many organic compounds give off invisible, harmful vapors that linger in stale air and irritate lungs. Any spill deserves full attention. Even a drop on the benchtop calls for a proper spill kit—not tissues, not water and a wish. My experience taught me fast: the downtime after a minor exposure slows progress far more than a careful, methodical set-up.
Secure Storage Routines
Unlabeled vials create confusion and accidents. Flammable or toxic solids like this one belong only in clearly marked, tightly sealed containers, away from light and humidity. Access should be limited to those who truly need it. I remember a trainee removing a chemical during a shift swap that hadn’t been logged; the resulting panic could easily have turned to disaster. Chemical inventories and check-in/check-out procedures may feel tedious but matter deeply.
Rethinking Disposal: Out of the Lab, Not Into the World
The trash bin or the sink cannot handle specialized waste. Years ago, I watched wastewater teams protest when local labs flushed organic chemicals, leaving them with an impossible cleanup downstream. These fluorescent markers can persist and affect aquatic life or leach into drinking water. Proper disposal means collecting every bit of solid and liquid waste, storing it in pre-labeled, compatible containers, and handing it over to certified hazardous waste handlers. Incineration under controlled conditions often provides the safest breakdown of organofluorine compounds—ordinary landfill or sewer systems fail to neutralize these persistent chemicals.
Practice, Protocols, and Accountability
Good intentions do not guarantee safe outcomes. Training sessions covering specifics—never just “general lab safety”—build muscle memory, so the right steps come naturally during mishaps. Regular drills help new and veteran researchers respond correctly to spills, fires, or exposure. Written procedures reviewed often, not left to gather dust, make all the difference.
Solutions and Culture Shift
A strong safety culture grows from collective responsibility. Team leads and lab managers need to model safe habits and call out shortcuts before they lead to harm. Investing in better detection and containment systems proves more cost-effective than facing cleanup costs or liability. Funding agencies and research institutes benefit by sponsoring regular hazardous materials training, ensuring that respect for the chemical doesn’t wane.
The Bigger Picture
The scientific community earns its reputation by not just pushing toward discovery, but stewarding safely every resource along the way. Knowing the risks, setting clear rules, and living those guidelines day-to-day builds public trust. Keeping harmful chemicals out of the environment and away from untrained hands protects both our work and our world, letting research move forward with confidence and integrity.