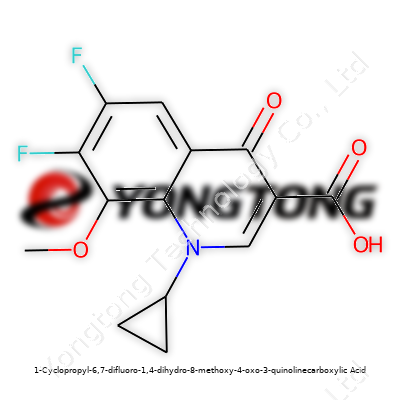
1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic Acid: Insight and Outlook
Historical Development
Chemistry often feels like following traces left behind by curiosity and necessity, and the path of 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid shows that spirit. The development of quinolone antibiotics changed the landscape of infectious disease treatment. In those early days, researchers aimed to improve potency and spectrum, moving from nalidixic acid and norfloxacin and pressing forward with fluoroquinolones. 1-Cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-3-quinolinecarboxylic acid emerged in the late twentieth century as chemists sought to fine-tune antibacterial power, reduce resistance, and make the drugs safer for wider use. The addition of methoxy groups, cyclopropyl rings, and fluoro substitutions didn't spring from imagination alone—these modifications were directed, based on how microbes evolved defense against earlier drugs. Medicinal instincts kept pushing for molecules that could outmaneuver bacteria and handle sturdy manufacturing processes as well.
Product Overview
Standing on the shoulders of earlier research, this compound brings together a cyclopropyl group at position 1, difluorine on positions 6 and 7, and a methoxy at position 8, fused with the standard 4-oxo-3-quinolinecarboxylic acid backbone. Those features make the compound a standout candidate for tailoring quinolone antibiotics. Having worked in research environments where these molecules are handled, I've seen how adding fluoro and methoxy groups can enhance activity against both Gram-negative and Gram-positive organisms, without easily falling to bacterial resistance. The substance marks progress, not just as a molecule, but as proof that chemistry keeps moving forward, one functional group at a time.
Physical & Chemical Properties
On handling, you notice a fine, off-white to light yellow powder that displays moderate solubility in water, better solubility in organic solvents such as methanol or dimethyl sulfoxide. A melting point ranging from 240°C to 247°C speaks to thermal stability, a trait valued during storage and pharmaceutical formulation. Fluorine atoms at positions 6 and 7 stiffen the aromatic system, boosting electron withdrawal and improving resistance to metabolic breakdown. The cyclopropyl ring increases lipophilicity, which helps the compound move across cellular barriers—a crucial feature when trying to reach infections deep in tissue. pKa values typically hover around 6.2, providing a balance between cell membrane permeability and water solubility. Experience shows that during weighing and transfer, the material does not cake easily and remains free-flowing, a help for scaling-up production.
Technical Specifications & Labeling
Quality control labs set high bars for identity, purity, and residual solvent content. Specifications usually demand at least 98% purity by HPLC, with impurity profiles well-documented and tightly controlled. Microbial limits, heavy metal content, and particle size distribution all get examined—years of seeing regulators peer into every detail of an API have shown me this keeps products from being sidelined by a single bad batch. Labels carry not just the molecule's full IUPAC name but also storage conditions—dry, cool spots away from sunlight—and batch traceability information. Safety data accompanies every container, from drums down to vials used for analytical testing, making it easier for anyone in the lab or plant to pull up exact hazard and handling guidelines.
Preparation Method
Synthesis follows careful choreography: construction of the quinoline ring, strategic introduction of difluoro, cyclopropyl and methoxy groups, and selective oxidation steps. The early steps may begin with a substituted aniline, passing through cyclization with keto acids, then forming the oxo-quinoline skeleton. Electrophilic fluorination, methyl etherification, and cyclopropyl ring installation each use well-established reagents and catalysts, but they call for precision. I've seen how process development chemists tinker with every temperature and reagent stoichiometry to maximize yield and minimize waste. Where early lab work used cumbersome routes with harsh conditions, newer protocols now cut down on hazardous intermediates, improving safety without sacrificing output. Robust purification—usually with recrystallization and sometimes preparative chromatography—delivers solid product that meets pharma requirements.
Chemical Reactions & Modifications
The molecule stays stable under mild acidic and basic conditions, but exposure to strong acids or bases, ultraviolet light, or oxidizers can trigger degradation. I've watched teams design derivatives: turning the carboxylic acid into esters for potential prodrug versions, or swapping the methoxy for other electron-donating groups to study the effect on antibacterial action. Fluoro substituents resist change, making the molecule steadfast during fermentation or downstream processing. Modifying side chains at the cyclopropyl or methoxy positions often yields new pharmacological properties, especially when trying to beat emerging bacterial threats or target hard-to-treat tissue infections.
Synonyms & Product Names
This compound often appears under a host of research code names, synonyms, and international nonproprietary names, depending on context. Besides its systematic designation, it's called by shorthand like CPDQM, difluoromethoxyquinolone acid, or simply referred to by the developing pharmaceutical company’s in-house number during early studies. Those codes may appear in patent literature or chemical databases. Sometimes confusion arises when different vendors or research publications pick subtly different nomenclature, which complicates internet searches or regulatory filings. In the lab, good habit says stick with CAS numbers or complete trivial names to sidestep mix-ups that can cost time or put a project at risk.
Safety & Operational Standards
Inhalation or ingestion risks add a layer of caution to routine handling. As a quinolone derivative, exposure can irritate eyes, skin, or respiratory tracts, so solid respiratory and skin protection belong in any handling protocol. The compound doesn’t ignite easily, but organic dust can accumulate static charge, which means grounding equipment and workbenches is smart practice. Waste from reactions or cleaning steps needs collection as hazardous chemical waste, underlining the factory rule: nothing down the drain, everything tracked till destruction. Experiences with regulatory audits reinforce the need for written SOPs, spill responses, and material safety sheets ready for inspection at all times. Driver training for hazardous material transport and lockable storage prevent theft or diversion into unauthorized settings.
Application Area
Modern healthcare would look different without the class of molecules this compound supports. Its main use remains in the pharmaceutical sector, feeding into the design and manufacture of second- and third-generation fluoroquinolone antibiotics. Research groups constantly evaluate its derivatives for newer indications—some have been checked against tuberculosis, sexually transmitted infections, and hard-to-heal wounds. In agricultural science, there’s a push to resist easy adoption, knowing resistance development in crops or livestock could undermine medicines for human use. Where I've seen collaboration work best is where public health experts, clinicians, and pharmaceutical developers sit together to map out where these molecules can do the most good without compromising future options.
Research & Development
Academic and pharma labs keep probing the molecule's potential. Structural tweaks at the methoxy and cyclopropyl positions keep generating candidates with better pharmacokinetics and reduced side effect profiles. High-throughput screening and computer modeling can now predict which modifications will likely produce promising antibiotic activity, conserving time and resources. I've watched machine learning algorithms narrow down which functional groups to try, shortening the guesswork phase dramatically. Preclinical studies explore not only efficacy but effects on the gut microbiome, minimizing collateral damage to beneficial bacteria. Studies track penetration into difficult tissues—bone, abscesses, lung—since some infections evade weaker drugs by hiding out.
Toxicity Research
Safety data always matters more than slick chemistry. This class doesn't escape scrutiny—neurotoxicity, tendon rupture, QT prolongation, and phototoxicity concerns all drive rigorous animal and cell-line testing. Repeated dose studies set clear thresholds for safe exposure, and regulatory toxicologists stay alert for subtle genotoxic or carcinogenic signals. In experience, sharing early data with regulators and independent toxicology boards limits costly roadblocks later in development. Some derivatives have lower toxicity in animal models, and companies hope those versions will translate to safer drugs for patients. Still, vigilance remains constant, shaped by tragic histories of drugs that fared well in labs but poorly in real communities.
Future Prospects
Resistance keeps rising, and the medical establishment craves new antibiotic options with improved safety and efficacy. Derivatives of this molecule—some already on the market, others deep in late-stage clinical trials—show real promise in treating resistant bacterial infections. Synthetic chemists and pharmaceutical partners can draw on decades of structure-activity relationship insights to quickly iterate new versions as resistance patterns shift. Investment in green chemistry to streamline manufacturing has grown, as both cost and sustainability pressure mount. In more forward-thinking labs, work begins early on environmental fate and residue control to keep pharmaceutical pollution from water and soil. Clinical guidelines, hospital stewardship programs, and cross-industry partnerships matter just as much as lab discoveries for these medicines to hit their full potential without triggering the next cycle of resistance.
Far From Just a Mouthful: A Closer Look
With a name that big, most folks would never guess the impact hidden behind all those syllables. This chemical, a part of the fluoroquinolone family, finds its place in medicine cabinets because of its antibacterial power. Harnessing the quinolone structure, scientists crafted a molecule meant to fight some pretty stubborn bacteria. Once a physician prescribes a medicine built on this compound, it targets bacterial DNA, stopping growth right in its tracks. I’ve seen patients perk up after a few days of the proper antibiotic—what seems like just another pill can bring real hope to a tough infection.
Medical Uses That Matter
This compound goes into drugs for tough-to-beat infections—think urinary tract, respiratory, and some skin infections. In clinics, these antibiotics matter most when older choices wind up failing or bacteria learn how to dodge standard treatments. The rise of drug-resistant germs gives real urgency to compounds like this one. I remember seeing cases in hospitals where other drugs lost their punch, and the doctor’s last lines of defense counted on molecules built from this chemistry.
Doctors rely on strong data confirming that these compounds attack a wide array of bugs. Studies have documented their action against E. coli, Klebsiella, and Pseudomonas—organisms that sometimes land folks in serious trouble. Having a broad weapon like this can save lives, especially when infections turn aggressive.
Why Risks Show Up Often in the Fine Print
Strength rarely comes without tradeoffs. People taking medications containing this compound sometimes deal with side effects—tendon issues, nerve pain, even heart concerns in rare cases. During pharmacy shifts, plenty of patients asked if antibiotics would hurt them more than help, and questions like that show how complicated the balancing act gets. The FDA issued safety warnings because these antibiotics, though potent, sometimes spark serious complications. That’s another reason physicians think hard before choosing this route.
Tackling the Threat of Resistance
Bacteria change constantly, and using antibiotics with a wide reach heightens the risk that germs adapt. Reports about rising antibiotic resistance trouble doctors and researchers alike. Overusing these drugs speeds up the arms race between medicine and microbes. Keeping this compound effective means using it only when absolutely necessary. Sharing decision power between patients and prescribers can keep these molecules in the toolbox longer. Getting clear about risk, refusing to use strong drugs for minor sniffles, and turning to lab cultures for guidance all play their part.
The Road Forward
Innovation never sleeps. Scientists look for smarter ways to use molecules like 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid, develop dosing strategies, and pinpoint which patients need them most. Supporting more research into alternative treatments and better diagnostics helps take some of the pressure off. Patients—armed with real information—can ask the right questions before picking up a prescription. Medical professionals who stay updated on resistance trends can catch problems before they spread. Caring for each other means protecting the medicines that still work and knowing which battles they are worth fighting.
Digging Into the Data
This chemical’s name is a mouthful, but for folks with a background in antibiotics, it rings a bell. It works as a building block in some fluoroquinolone drugs. Doctors have handed out fluoroquinolones like ciprofloxacin and levofloxacin for decades to treat infections. That history doesn’t guarantee safety. People still need to ask tough questions, because lab results and life can tell two different stories.
How It’s Used
Scientists know that fluoroquinolone drugs help fight nasty bacteria, including the sort that shrugs off older medicines. Drug makers tinker with the core structure, swapping out chemical bits to boost killing power and dial back side effects. 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid sits in the middle of this chemical shuffle. On its own, it isn’t pumped into syringes or pressed into pills. Still, anything that lands in a bloodstream — even by accident — matters.
Looking at the Risks
No journey into drug safety makes sense without data. Fluoroquinolones aren’t strangers to problems. Some trigger tendon injuries, nerve pain, mood swings, or blood sugar drops. That’s not just gossip; the FDA stuck a “black box” warning on these drugs. This chemical forms the backbone of many such medicines, so I approach it with a sense of caution.
Animal studies and toxicology reports watch for red flags — organ damage, genetic changes, and the like. Data on pure 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid remains sparse. Most experiments lump it into a bigger soup, checking its power in fighting bugs more than its solo safety. That doesn’t answer every question. Absence of proof isn’t proof of safety.
Why Oversight Matters
Lab mishaps, factory leaks, or online sales can move such chemicals outside their intended purpose. Untrained folks trying to cook up “DIY” antibiotics worry me. That’s not just about following regulations — it takes training to work safely with reactive or toxic chemical powders. A tiny lab accident teaches hard lessons.
A few years back, a local university cleaned up a spill from a research project. Hazmat teams wore layers of gear. Even with small exposures, some staff felt dizzy or got skin rashes. Emergency rooms rarely see cases like that, so the right protocols matter.
Safe Use and Trusted Sources
Pharmaceutical companies making regulated drugs track every step, from ingredient testing to final packaging. Quality control labs check for impurities and cross-contamination. Those practices don’t exist in a hobbyist’s kitchen. That gap lies at the heart of every “is it safe?” debate.
For patients, doctors make dose decisions based on years of research and experience. At home, even a tiny measuring error can bring harm. Stories of antibiotic resistance or toxic reactions from imported pills only underscore these risks.
Protecting Health
My own view lines up with experts: stick to medicines made and dispensed through regulated channels. Anyone working with research chemicals needs training, protective gear, and solid protocols. People who feel sick or need antibiotics should steer clear of shortcuts — health matters too much to roll the dice with unknowns.
Tough oversight, careful quality checks, and honest information give people the best shot at staying safe.
Recognizing What’s at Stake with Powerful Antibiotics
1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic Acid belongs to the fluoroquinolone class. Doctors use drugs in this family to tackle tough bacterial infections, a decision not taken lightly. Patients deserve to know what risks they might face. Too often, people assume something prescribed in a hospital will only help. The full reality looks different.
Short-Term and Obvious Side Effects
Many fluoroquinolones bring clear physical reactions. Gut trouble like nausea or diarrhea shows up in more folks than most realize. Headaches, dizziness, or a strange taste in the mouth get reported often; it’s not just a rare thing for a few people. When I helped care for someone taking this class of antibiotics, they needed warning about sunlight too. These drugs can make skin more sensitive to burns. Some patients get a rash or redness after just a little sun, and they chalk it up to weather or allergies without connecting the dots.
More Serious Risks: Nerves, Tendons, and Mental Health
Over the years, it has become clear that a small but real number of people suffer serious side effects with these drugs. Tendons get hit hard—Achilles tendon problems come up so often that warnings sit right in the labeling. Some people experience tendon pain, swelling, or even rupture, especially if they happen to play sports or move around a lot on the job. Older age and use of steroids raise this danger.
On the nerve side, the FDA reports cases of neuropathy—burning, tingling, or numbness in the legs or arms that sometimes never stops. These effects may appear quickly. I’ve seen a friend struggle with months of strange sensations that outlasted the infection itself. Paying attention to those early warning signals—the pins-and-needles, the sudden muscle weakness—makes a huge difference in minimizing long-term harm.
The mental side matters too. Agitation, trouble sleeping, anxiety, and confusion hit some users, sometimes on the first day. Not long ago, a neighbor talked about feeling detached and irritable while on a course of quinolones, chalking it up to the stress of illness. We need to highlight to patients that the medication can feed these feelings, and at times spark real psychiatric problems.
Organs, Blood Sugar, and Drug Interactions
The risk to organs shows up in some who start these drugs. Liver enzymes rise—sometimes quite a lot. Some users notice yellowing skin or eyes, a warning sign for hepatitis. The kidneys work overtime clearing this compound, so anyone with kidney problems needs extra monitoring. Blood sugar swings catch a few people off guard as well, with both lows and highs possible. Diabetics deserve extra care and might need to check their sugar more than usual during treatment.
Interactions with other medicines present another set of challenges. Drugs to thin the blood (like warfarin), medications for heart rhythm, and even common supplements have reported issues. Doctors and pharmacists should walk through these risks instead of relying on computer warnings.
Staying Safe with Science-Led Choices
The bottom line: 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic Acid serves a purpose when other antibiotics fail or resistance leaves few options. Using antibiotics in this group should never be routine. Open talk between prescribers and patients about risks—based on hard evidence—is non-negotiable. If a course gets started, watch for side effects. Early reporting and switching medications at the first sign of trouble can save lasting harm. Bringing real-world patient experiences into these conversations helps us learn from each other and keep as many people safe as possible.
Sources include peer-reviewed medical journals, FDA safety updates, and lived experience working with patients on antibiotics.
More Than Just Chemistry: Human Impact Matters
People see chemical names like 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid and think: scientist’s business. Truth is, dosage touches on daily life for anyone who needs antibiotics, since this molecule sits inside the fluoroquinolone class, a group used to tackle tough bacterial infections. I spent three months on antibiotics for a rare sinus infection. Doctors didn’t just guess at my dose. They made choices based on my body, risk of side effects, how my kidneys were handling life, and the stubborn bug growing in my head. Watching how the right dose changed everything for me—good and bad—gave me a lasting respect for the science behind these numbers.
Facts Make a Difference: No One-Size-Fits-All Dosage
The urge to google the “correct dosage” for a complex medicine pops up fast. Regulatory guides like FDA and European Medicines Agency don’t offer a blanket dose here. They say: check the patient’s specific needs, infection type, age, liver and kidney health, sometimes even DNA quirks. Fluoroquinolones as a group, which include this compound, hit hard and dig deep, but they also carry risks—think tendon ruptures, nerve pain, mental cloudiness.
Clinical studies—reviewed by panels of infectious disease experts—suggest a range for most adults, but these numbers only work when plugged into a larger clinical picture. For example, ciprofloxacin (a related medicine) can run 250 mg to 750 mg twice daily, though serious infections might require IV forms or different schedules. Direct swaps or home calculations don’t make sense, because changing a single group on the molecule can alter its action. No published guidance yet proves that this compound should be given at the same dose as its relatives. That’s something only randomized, peer-reviewed trials and regulatory review will solve.
Risks Lie in Both Scant and Excessive Dosage
Go too low with the dose—bacteria play dead, return stronger, and doctors soon face antibiotic resistance. Go too high—increased risk of ruptured tendons, seizures, or permanent nerve pain. Older folks, those with kidney or liver troubles, and people on other medication need extra caution. Depersonalized dosing turns medicine into roulette.
Stories from the ER keep rolling in: someone with a complex infection gets a huge dose, doesn’t tell their doctor about their blood pressure meds, and winds up dizzy, falling, or worse. Others underdose, thinking it’s safer, and end up back in the hospital with an infection that laughs at standard options.
Potential Solutions: Careful Evaluation Changes Everything
Smart solutions grow from good communication: patients share everything they can about their health, allergies, and medicine history; doctors use the latest guidelines and up-to-date clinical data. Hospitals should adopt stewardship programs, where a pharmacist or infectious disease specialist checks dosing plans before the first pill goes down. Electronic health records with allergy alerts and kidney/liver function reminders can help.
Clinical trials still need to run their course before anyone can say this specific molecule has a gold-standard daily dose. Transparency from pharmaceutical companies, charitable research funding, and clear guidance from regulators will nudge science toward safer, smarter answers.
Space for reflection matters—each dose change is more than math. It means sitting with the real consequences of getting medicine right or wrong, and remembering that behind every prescription, there’s a living person relying on the people writing those orders.
Understanding the Substance
There’s something at stake when medicines punch above their weight in chemical complexity. 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid is a mouthful on the label, but in practice, it falls in a family closely related to many fluoroquinolone antibiotics. Folks in healthcare circles know drugs of this type can save lives in stubborn infections. With complicated names like this, we approach them with some healthy caution, especially when patients line up other pills on their kitchen counter.
What Drug Interactions Mean in Real Life
I remember a summer rotation in a small-town pharmacy, counting out different pills for someone taking a cocktail of prescriptions. Drug mixing doesn't happen abstractly in a chart. Patients stack prescription bottles on their nightstands. They forget to bring a list to every visit. Nobody wants an extra trip to the ER because of a run-in between medications.
Fluoroquinolones, and similar structures to this acid, often have run-ins with other medicines. Common culprits include antacids, blood thinners, and some diabetes drugs. Calcium, magnesium, and iron can tangle up with quinolones and block their absorption — the medicine doesn’t break down where and when it should. It’s not just a theoretical risk. People routinely toss back a calcium-fortified orange juice or swallow a vitamin without thinking, which can cut the punch of their infection-fighting prescription.
Evidence and Risks
Let’s take it from what’s proven. Fluoroquinolones as a group have flagged interactions. Anticoagulants like warfarin have gotten in trouble when paired with them, raising the risk of unexpected bleeding. Blood sugar swings have hit people taking certain diabetes drugs, which mix poorly with these antibiotics. No one has to rely on rumor here; FDA safety reports, real-world hospital records, and published research back up these concerns. It’s not a rare or trivial problem.
I once watched an older neighbor end up with tremors and confusion because his infection and diabetes weren’t handled with a careful eye for drug interactions. The answer was hiding in plain sight: two prescriptions written days apart, no head’s up about how they might fight in his system.
Addressing the Problem
Tackling these issues comes back to communication. Any time a doctor considers prescribing anything in the family of 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid, patients need a full run-down of what they’re already taking, including supplements and over-the-counters. Pharmacists ask about vitamins, but that only works when patients remember everything — or feel comfortable sharing. Every pill, powder, or drink with added minerals opens a possible interaction.
Solutions rely on better records and transparency. Electronic prescription systems that flag drug interactions help. Regular check-ins, not just at initial diagnosis but every time the medicine list changes, catch problems early. Patients deserve a chance to ask about new symptoms, not just get a quick nod at the counter. Health literacy campaigns that turn technical warnings into plain talk build trust, too.
Moving Forward with Open Eyes
Mixing potent antibiotics, like any quinolone derivative, with a daily routine full of supplements, heart pills, and antacids creates a challenge that pharmacists and physicians tackle every day. Real risks call for clear caution and honest conversations. I’ve seen the best outcomes come when patients have space to share, ask questions, and take their medication plans as seriously as their doctors do.
References:- FDA Drug Safety Communications
- Clinical Pharmacology Databases
- Peer-reviewed journals such as the Journal of Antimicrobial Chemotherapy