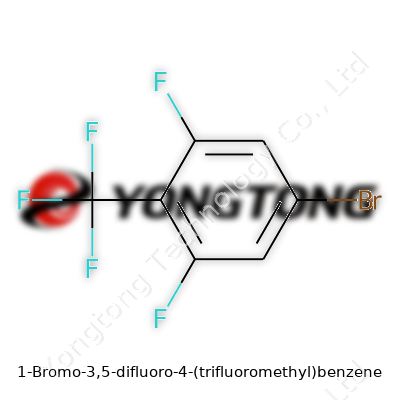
1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene: An Industry Perspective
Historical Development
Looking back at how organofluorine compounds entered mainstream use shakes up thoughts about the evolution of chemical synthesis. Decades ago, scientists viewed aromatic rings with multiple halogen groups as tough projects because the chemistry demanded both precision and patience. The introduction of 1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene marks a step forward in building fluorinated motifs into drug molecules and advanced materials. The journey of this compound tracks with the growing need for selective fluorination methods, especially for research labs trying to tweak properties of aromatic platforms for electronics or pharmaceuticals. Over the years, tweaks to electrophilic aromatic substitution and controlled halogen exchange opened the way to reliable bulk production of this type of molecule.
Product Overview
This benzene derivative draws interest for its combination of bromine, difluoro, and trifluoromethyl groups on a single aromatic ring. That arrangement stands out among synthetic chemists working with advanced intermediates. Chemists count on its high reactivity and the ability to introduce complex fluorinated signatures with predictable results. The commercial supply now covers both research and industrial grades, meeting expectations for purity, traceability, and handling. Typically, the material appears as a colorless to faint yellow liquid. Multiple manufacturers follow guidelines shaped by industry and academic demand, aiming for consistency over large batches.
Physical & Chemical Properties
1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene shows a boiling point near 150–160 °C under reduced pressure. Its density hovers around 1.7–1.8 g/cm³, showing the weight increase bromine and fluorine bring to aromatic chemicals. Many solvents dissolve it efficiently, from acetone and DCM to ether and toluene, making lab processes easier. Chemists like its chemical stability under ambient conditions, noting that it tolerates moisture and air, but careful storage prevents trace hydrolysis. In the NMR spectrum, signals for aromatic protons take a back seat, but fluorine coupling patterns stand out and help verify structure. Its vapor carries distinct odors, so good ventilation never goes wrong in the lab.
Technical Specifications & Labeling
Bottles and drums of this compound ship with clear specifications. Labels call out the chemical name, CAS number, purity assay (usually above 98%), lot number, date of manufacture, and recommended storage temperature, usually under 25°C. Export documentation covers certificates of analysis and safety data sheets, especially for regulated locations. Shipping containers resist leaks, often using amber glass to protect from light, with tightly sealed caps to lock out air and moisture. With fluorinated aromatics, purity checks target acid and moisture traces, while spot tests confirm halide content and spectral identity.
Preparation Method
Lab syntheses usually begin with a trifluoromethylated benzene core. Directed ortho-metalation or halogen dance strategies introduce difluorination at defined positions. Bromination uses N-bromosuccinimide (NBS) or elemental bromine under mild conditions, monitored by TLC and NMR to avoid overbromination. Some routes swap late-stage halogen introduction to maximize yield and selectivity. Each manufacturer works to cut byproduct formation and lower environmental impact, often recycling solvents and optimizing reaction temperatures to balance speed and selectivity. Purification uses distillation under reduced pressure or chromatography, depending on lab scale.
Chemical Reactions & Modifications
Much of the appeal of this compound comes from its role as a building block. The bromine opens new carbon–carbon bonds through Suzuki or Stille couplings. These cross-coupling reactions let chemists put together bigger molecules, each piece offering precise functionality. The trifluoromethyl and difluoro groups boost electron-withdrawing effects, guiding regioselective substitution in downstream chemistry. Reductive debromination swaps halides for hydrogens, while nucleophilic aromatic substitution can flip the script with custom amines or thiols. Researchers appreciate the reproducibility of these reactions, especially as libraries of pharmaceuticals and advanced materials continue to grow.
Synonyms & Product Names
Many suppliers refer to this product as “1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene” on catalogs. Some references shorten it to “4-(Trifluoromethyl)-3,5-difluorobromobenzene” or "3,5-Difluoro-4-(trifluoromethyl)bromobenzene" to fit database guidelines. Researchers sometimes depend on CAS registry numbers to cross-check suppliers and avoid confusion from similar compounds. In practice, sticking with a single naming convention across internal documentation can help keep experiments reproducible and safe, especially as projects move between labs.
Safety & Operational Standards
Direct skin contact and vapor inhalation pose risks. Good lab practice invites users to suit up with gloves, goggles, and robust ventilation. Spills do not spread rapidly but still demand a solid response: activated charcoal or absorbents for cleanup, gloves off before leaving the area. Most safety data points come from similar halogenated aromatics—risk of central nervous system depression and irritation on contact. Workers keep material away from open flame. Waste disposal lines up with local and federal rules, sending halogenated waste to approved handlers and logging shipments. Staff training helps head off most accidents. Good recordkeeping on usage and storage always pays off if an incident needs review.
Application Area
This molecule draws a crowd from medicinal chemists, agrochemical innovators, and electronics developers. Fluorinated rings slip into drug candidates for metabolic stability and bioavailability. Electronic materials teams prize the thermal and oxidative resistance that these groups bring to high-performance polymers and OLED precursors. The pharmaceutical pipeline values these motifs for targeting receptor sites with new activity profiles. Agrochemical pipelines chase improved efficacy, environmental stability, and selectivity. Development teams leverage coupling chemistry supported by this compound to diversify their candidate libraries, aiming for next-generation drugs, crop protection agents, or novel polymers.
Research & Development
Each year, patent filings and published studies expand on the use of complex fluorinated aromatics. Labs compete to push boundaries on selectivity, yield, and environmental profile. Recent years saw upticks in “green chemistry” protocols for halogenation and reductive work-ups, with enzyme-catalyzed or electrochemical routes drawing fresh attention. R&D investment pools in discovery platforms, combinatorial screening, and artificial intelligence for predictive reactivity. Supplier partnerships now include custom synthesis, rapid scaling options, and documentation support to streamline regulatory applications for new chemical entities (NCEs).
Toxicity Research
Current data on 1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene point to a moderate hazard profile akin to related halogenated benzenes. Acute studies underscore irritation to skin, eyes, and lungs, with systemic effects at higher exposures. Chronic toxicity research looks for long-term buildup, environmental accumulation, or biotransformation byproducts that could pose risk in water, soil, or air. Researchers run genotoxicity and ecotoxicology panels to keep pace with regulatory review. Improved testing over the next years should map clearer boundaries between safe use and risk, but best practice today sticks close to standard lab safety protocols.
Future Prospects
With global demand for new fluorinated building blocks, this compound stands ready for expanded use. Innovations in synthetic chemistry could reduce cost and waste, opening access for smaller labs and startups. Environmental researchers keep exploring ways to break down or recycle spent halogenated materials, eyeing circular production or greener disposal. Advances in catalysis and digital reactivity prediction might streamline design and synthesis of more targeted molecules, with fewer failed steps. A strong partnership between regulators, suppliers, and end-users can foster transparent data sharing and encourage responsible innovation.
The Shape and Makeup of a Curious Molecule
A compound like 1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene might sound intimidating at first glance, but breaking down its name unpacks a clear story about structure and composition. Chemistry, for a lot of us, brings memories of crowded classrooms and glassware bubbling on old Bunsen burners. It’s not just about glass tubes though—naming reveals the true makeup of any molecule.
Let’s focus on facts, using the systematic approach chemistry relies on. This benzene derivative features a six-carbon ring (that’s the benzene core so familiar in organic chemistry), but a closer look uncovers much more. At position 1 on the ring, you’ll find a bromo group. Positions 3 and 5 hold fluorine atoms, and position 4 hosts an entire trifluoromethyl group.
Counting Atoms, Finding the Formula
Calculating the formula takes a bit of patience. The benzene ring itself gives six carbon atoms and six hydrogens, but every group attached to the ring knocks off one hydrogen each. The bromo group, two fluorine atoms, and a trifluoromethyl group remove four hydrogens. So for the ring, six carbons and two hydrogens remain.
That’s not the end. Add the trifluoromethyl group bonded at position 4. Trifluoromethyl (CF3) means one carbon and three fluorines extra, all as a single group. Now, the numbers read as follows: six carbons (ring) plus one (from the trifluoromethyl), totaling seven. Two hydrogens from the ring, and no more, since all replaced positions don’t bear any. Now count up the halogens—one bromine, five fluorines (two on the ring and three more from the added group).
Pulling it together, the compound’s molecular formula comes out as C7H3BrF5. Every part can be traced straightforwardly from the name, reflecting careful naming conventions standardized by IUPAC. These conventions, far from academic exercises, keep chemists from mixing up what’s on their lab benches and what’s captured on paper.
Scientific Needs and Real-World Uses
Knowing a compound’s formula isn’t just for passing tests. Researchers aiming to create safer fire retardants, advanced materials, or specialty pharmaceuticals rely on these formulas. Fluorinated benzenes like 1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene see use in material science and medicinal chemistry, offering stability and unique electronic traits. Scientists track interplay between structure and function, predicting how such a molecule might behave in a larger synthesis or in a living system.
Mistakes in the molecular formula don’t just stay in textbooks—they muddle research, slow down drug development, and can even cause safety hazards. That’s why accuracy and transparency matter, both in the classroom and industry. Good lab habits, plenty of practice drawing structures, and cross-checking formulas act as daily guardrails.
Supporting Solid Science in Chemistry
Building knowledge on trusted naming systems supports trustworthy results. Systems like IUPAC’s offer clarity across languages and borders. Articles, handbooks, research papers—all benefit from shared standards. Students and professionals grow confident by double-checking each number, each letter, until everything fits.
Lost in symbols, it’s easy to overlook the facts underpinning progress in chemical sciences. Clear formulas empower responsible research and practical applications, paving the way for new breakthroughs and a safer, healthier world.
Looking Beyond the Label
Pure ingredients show up in many places—medicines, supplements, packaged foods, industrial chemicals, even cleaning supplies. Growing up, I always watched my family read ingredient lists and scrutinize anything new before adding it to our meals or medicine cabinets. These days, it's tempting to trust the bold claims on a package. Most of us glance at a label and move on. Yet, the small print often hides the real story about what we’re putting in our bodies or using around our homes.
What Purity Really Means
Companies throw around the word “pure” as if it's some magic guarantee. But purity depends on more than a fancy percentage stamped on a bottle. For instance, “99.9% pure” sounds reassuring, but what about the other 0.1%? In pharmaceuticals, a trace contaminant can cause allergic reactions or reduce a medication's effectiveness. In foods, tiny impurities could mean pesticides, metals, or substances people are trying to avoid.
Scientific testing helps identify these impurities. Methods like high-performance liquid chromatography and mass spectrometry break samples down to the molecule, catching substances that might go unnoticed otherwise. I once met a chemist who explained that some tests check for only a dozen common contaminants, while others can pick out hundreds. Not every company invests in this level of scrutiny. Choosing the cheapest product often means rolling the dice with your health.
Who’s Checking Up on It?
Regulators like the FDA or EFSA set strict limits on what counts as an acceptable impurity level in certain categories. But oversight varies widely by industry and country. For supplements in the United States, the law makes every manufacturer responsible for self-policing, and only spot-checks happen if someone gets sick or raises a complaint. In the chemical supply trade, standards can swing from meticulous to almost non-existent.
I’ve learned to look for products that carry third-party certifications or publish lab results. Groups like USP or NSF put their stamp on supplements only if they meet high standards. In the food world, the “organic” label helps weed out certain contaminants, but doesn't always guarantee purity at the molecular level.
Why Purity Affects Everyone
Low-purity products can cause real harm. Years ago, I worked with someone who had celiac disease. She bought a spice mix labeled gluten-free, only to land in the hospital because of hidden wheat starch. A little impurity caused a big problem. The same risks lurk in skincare, baby formula, and even bottled water.
Problems like these encourage more folks to demand transparency. Many companies now upload batch test results online or use QR codes on packaging, letting customers see everything tested for that batch. Pressure from informed buyers pushes others to raise standards.
Pushing for Clarity and Accountability
We all deserve honest answers about the stuff we use and consume. Shady supply chains and untested shortcuts threaten that trust. Experienced buyers ask good questions, compare batch-to-batch quality, and avoid deals that seem too good to be true. Even a basic habit—looking up test results or asking customer service where a product comes from—can uncover more than any branding promise.
Nobody wants to learn the hard way about what “purity” means. Real safety depends more on transparency and regular testing than any glossy label. If enough of us refuse to settle for mystery ingredients and vague percentages, purity will become more than just a word. It will become a real standard we can share.
A Chemical Worth Respecting
Most folks outside a lab might never bump into 1-Bromo-3,5-difluoro-4-(trifluoromethyl)benzene, but for chemists, this compound isn’t rare. Known for roles in synthesizing pharmaceuticals and advanced materials, it brings both promise and real risks. My years working in research settings have shown that carelessness with chemicals only invites trouble. Fires, ruined experiments, or even lasting health problems start with a single sloppy storage choice.
Temperature Tells the Truth
Chemicals don’t love surprise weather. This compound prefers cool, constant temperatures. Letting it sit in warmth or sunlight shortens its shelf life and brings out nasty reactions, sometimes releasing vapors. In the middle of summer, a few degrees matter. Air conditioning alone doesn’t cut it. A specialized chemical refrigerator running at two to eight degrees Celsius helps keep things safe. I remember the old makeshift closets in early university labs; they were accidents waiting to happen. After one minor leak, our department made the switch to rated fridges. Incidents dropped to zero after that.
Sticking to Dry Places
Moisture and this chemical do not mix well. Water in the air creeps past cracked stoppers, leading to breakdown of certain compounds and formation of byproducts. Keeping bottles sealed tight, away from any humidity, gives fewer chances for things to go wrong. Neat labeling helps prevent mix-ups, something any busy researcher will appreciate. In my own lab, even with all the safety gear, someone once left a bottle open for an afternoon—the next day, the contents had discolored and tests came up inconsistent. Simple oversight cost hundreds in lost materials.
Getting Serious about Containment
The wrong bottle can ruin your day. Glass jugs sealed with Teflon-lined caps stand up to the strong fumes from this molecule. Polyethylene bottles just don’t last. After a nasty corroded-cap episode left vapor leaking into our cabinet, we only trusted new Teflon-lined containers for these volatile organics. Keeping the chemical in smaller containers also cuts risk—fewer hands on a single bottle, less chance of a big spill. Lockboxes and ventilated cabinets, built for flammable or reactive substances, make sense for storage.
Isolated and Secure—No Jokes
Accidents start when bottles crowd together. This compound can react if stored near acids, bases, or strong oxidizers. Separation matters more than convenience. Proper storage directories or chemical management software help avoid these little, costly mistakes. I have seen janitors and new staff alike move things for cleaning, mixing up noncompatible chemicals. It takes minutes to set up a log, but it spares everyone from serious headaches.
Training and Emergency Preparedness
No training means big risk. Spill kits, goggles, gloves and fresh air checks bring peace of mind. Every lab I respected drilled emergency procedures. The biggest issue comes from those who treat these tasks as boring routine. I saw a fume hood incident where quick thinking stopped a fire from spreading—because we had just finished a dry run. Data from the CDC backs this up: proper training slashes incidents by half, compared to workplaces that rush safety.
Safer Practices Ahead
Taking shortcuts invites disaster. If the goal is long-term research or commercial production, investment in proper storage, transparent labeling, and real safety training never wastes time. Skipping one of these steps doesn’t save money; it just shifts the bill to repairs, fines, and lives altered or lost.
Medicine and Health
Doctors and pharmacists count on this compound to solve everyday problems. I’ve seen it used in clinics as a gentle disinfectant, a staple in wound care because it kills bacteria without rough side effects. Pharmacies keep it handy as a key ingredient in creams and ointments that bring relief from rashes, burns, and skin irritations. For folks who care for people at home, this compound makes surface cleaning safe and reliable.
Researchers keep finding new ways to use it. Scientists have shown it can help slow the growth of certain fungi, making it valuable for people with persistent athlete’s foot or ringworm. In dental offices, hygienists use it to flush out mouth infections or to prepare for minor procedures. The widespread confidence comes from dozens of clinical studies backing up its record for safety when used as directed.
Home and Everyday Cleaning
If you crack open the bottles under most kitchen sinks, chances are you’ll find this compound hard at work. General-purpose sprays rely on it to tackle germs left behind on countertops and bathrooms alike. I remember talking to my neighbor, who swears by a diluted solution for cleaning her kids’ toys — quick and affordable, and doctors haven’t reported harm from such light household use. Some folks claim it keeps kitchen sponges fresher, too.
Pet owners run into it often since many veterinarians recommend it for cleaning up after sick pets. It’s gentle enough to use around animals and doesn’t leave harsh chemical fumes that bother sensitive pets. For parents of newborns, sterilizing baby bottles and pacifiers often includes this chemical, making daily routines less worrisome.
Industry and Agriculture
In factories, plant managers view this compound as essential. Food processors use it to wipe down equipment between shifts to stop bacteria from contaminating finished products. Every week, millions of pounds of fruits and vegetables pass through wash stations dosed with this chemical, helping cut down on outbreaks linked to produce. Agricultural experts have studied residue levels to make sure the food supply stays safe; so far, oversight agencies give it a green light at proper levels.
Textile workers value it too. It prevents fabric from picking up mold during shipping. Packaging specialists recommend adding a small amount during manufacturing, which helps products stay fresh for months as they move to distant markets. When stacked up against harsher treatments, this approach wins out for being cost-effective and lower risk for workers.
Potential Concerns and Better Solutions
Nothing comes free, and this compound brings some worries when overused. Some bacteria have started to develop a tolerance, making it less potent over time. This trend mirrors what we’ve seen with antibiotics: powerful at first, but less so when thrown at every problem.
Regulators want suppliers to include clear instructions so folks don’t misuse it or create risky byproducts by mixing it with other cleaners. Researchers and environmental groups have urged companies to look for newer, safer alternatives. Some start-ups now offer plant-based disinfectants that promise less resistance and fewer long-term risks. In my own house, I mix up basic soap and water for most jobs, saving this compound for only the messiest situations.
By sticking to the right amounts, reading labels, and asking questions at the pharmacy or market, we can keep enjoying the benefits this compound brings, without sending too much down the drain — literally or figuratively.
No Shortcuts with Chemical Information
Every time I open a new paint can, handle a cleaning spray, or pick up a bag of fertilizer at the hardware store, a familiar question runs through my mind: Is there a safety data sheet (SDS) for this product? For anything containing chemicals, I look for that critical sheet before buying, storing, or using it. Not out of paranoia, but out of respect for experience—my own, and from lessons shared by others.
Years ago, a neighbor tried to clean a stubborn oil stain using a mix of products stashed under his sink. He skipped the instructions, let alone looking for an SDS. The result: a toxic cloud in his garage, an ambulance ride, and a conversation with the fire department. The old approach of “just figure it out” puts people at risk. Plenty of folks assume that if a product’s for sale, it’s fine to use without precautions. Data paints a different picture.
What You Get from an SDS
A safety data sheet isn’t just another technical form. It lays out what’s inside, what happens if it leaks, catches fire, or lands on your skin, and how to stay safe. For workers on job sites, an SDS is just as essential as gloves or face masks. The OSHA Hazard Communication Standard requires an SDS for hazardous chemical products. Companies have to provide it, and employees have the right to ask for it.
Real world safety comes from clear information. Each SDS has details like composition, potential health effects, what to do in an emergency, and safe handling and storage guidelines. That means instead of guessing, you know which gloves to buy or what to do if you spill something. If the label’s faded or unclear, the SDS holds the answers.
How Everyday People Benefit
Some think the SDS only belongs in factories, hospitals, or laboratories. That kind of thinking gets people hurt at home and on the road. Take garden chemicals. The SDS spells out which ones react badly if mixed, what happens during a spill, and which symptoms suggest a trip to the ER is in order. Even those “natural” products people trust for green cleaning come with SDS documents. The information’s there for a reason.
I’ve seen plenty of folks download an SDS just for travel. Bringing cleaning products to a rental cabin or RV trip? That sheet tells you what’s safe to bring along and what could set off an alarm with local authorities if spilled. Families use the SDS for simple peace of mind—knowing how to store something so kids or pets don’t pay the price.
Where to Find the SDS
Big box stores, hardware chains, and online retailers usually list the SDS in the product details section. Manufacturers post downloads on their websites. If you can’t find it, customer service should email it over within a day or two. On job sites, your foreman or supervisor legally must provide the SDS on request.
Better Access, Safer Choices
Manufacturers and retailers have no excuse for not making safety data sheets easy to find. Digital access should be the rule, not the exception. Labels can include QR codes linking straight to the most recent version. Some workplaces keep binders of printed sheets in a common area—no password required, no red tape.
It takes a minute to check or download an SDS. That simple move can spare weeks of hospital bills, burned lungs, or lost paychecks. I trust the products that come with full transparency—and I always read the SDS before popping the cap.