1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene: Commentary and Insights
Historical Development
Chemistry often advances through a mix of necessity and curiosity. 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene has a name that fills up almost a whole line on a notepad, but its story began with the rising demand for aromatic compounds that can weather tough reactions. In the eighties and nineties, synthetic organic chemistry started to lean hard into halogenated benzenes because they offered new handles for coupling and substitution reactions. Diaryl ethers, fluorinated aromatics, and similar compounds all drove competitive research among chemical suppliers and academic labs. Sometimes, just tossing in a couple of fluorine atoms made the difference between a compound abandoned in a dusty drawer and one that triggered a round of patents. Over time, researchers zeroed in on ways to combine bromine and fluorine on the same ring, along with groups like trifluoromethoxy that catch your eye both on paper and in labs. The result—molecules like this one—found a place not through blockbuster fame, but from quiet utility in everything from medicinal chemistry to electronics.
Product Overview
1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene isn’t the sort of chemical you’ll find in a household under the sink. It lands in the catalogs of specialty chemical vendors and catches the attention of synthetic chemists, especially those looking to build up libraries for drug or material science research. It’s tailored for people who need a reactive aryl bromide no stranger to aggressive reagents. You sense its main appeal when chasing molecules that resist metabolic breakdown, since fluorine atoms slow things down for the enzymes eager to chew up organics. Engineers and scientists use it as a starter block in reactions that introduce complexity onto an otherwise stable benzene ring. It doesn’t claim versatility beyond this sort of specialty chemistry unless someone, somewhere, discovers a weird new use in the next breakthrough paper.
Physical & Chemical Properties
Standing over the beaker, you notice that this compound forms a colorless to pale yellow liquid, not much different at a glance from plain organic solvents. The details matter more. With a melting point lying well below room temperature, you end up with a liquid at standard lab conditions. The boiling point stretches higher than many organic liquids, reflecting the sturdy, electron-rich benzene ring and the heavy bromine atom keeping volatility in check. Its density tips above that of water. The strong carbon-fluorine bonds, along with a bulky trifluoromethoxy group, push its lipophilicity upward and grant low polarity, so don’t expect water to dissolve much of it. Most chemists reach for organic solvents such as dichloromethane, ether, or toluene to get it moving or to dissolve it for further reactions. The reactivity stays centered on the bromine—a good leaving group that enables palladium-catalyzed coupling—and the electron-withdrawing groups tune the ring’s responsiveness.
Technical Specifications & Labeling
Chemicals like this don’t come without a pile of paperwork. Purity matters, and the best suppliers back up their certificates with data—sometimes NMR, sometimes GC-MS, always a batch number for tracing. Acceptable purity hits 97% or above, though certain applications demand even tighter control. Minute differences in structure spell a world of difference when chasing bioactivity or electronic behavior. Labels straight from the supplier usually list systematic names, any relevant safety signal words, batch IDs, expiration dates, storage instructions, and often a QR code linked to safety data sheets. Tracking material as it jumps between storage, lab, and waste stream stays essential both for regulation and safety.
Preparation Method
In a synthetic chemist’s hands, the journey starts with a suitably fluorinated or brominated benzene precursor. One common approach involves introducing the trifluoromethoxy group by nucleophilic substitution, where activated fluorobenzenes react with trifluoromethyl ether sources under heat and pressure. In many labs, metallation-wise, the starting aryl bromide arrives first, with fluorine added through selective fluorination—sometimes in the form of Selectfluor or other fluorinating agents. Stepwise protection and deprotection cycles, classic anhydrous conditions, and a careful purification process—silica gel, flash chromatography, or even distillation—mark the safest route to a product that doesn’t carry more by-products than desired. Even for a single compound, small tweaks to temperature, solvent, or reagent order can drive yields from pitiful to practical.
Chemical Reactions & Modifications
Chemists rarely use 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene straight out of the bottle. More often, it serves as a starting scaffold ready for Suzuki or Buchwald-Hartwig reactions since the bromine readily jumps off when you bring in a catalyst. The electron-withdrawing fluorines and trifluoromethoxy block off certain positions for further aromatic substitution but also provide useful selectivity. Crafty researchers know you can build more complex molecules by leveraging the precise reactivity imparted by each substituent. Hydrolysis, reduction, or nucleophilic aromatic substitution reactions sometimes get tried, although the barrier to displacement on the fluorinated ring runs pretty high. The chemistry stays practical rather than flashy—the kind of workhorse molecule used when one piece of a puzzle needs careful tuning.
Synonyms & Product Names
Catalogs and chemical databases pile up the synonyms until finding the right compound becomes a small research task on its own. In this case, you may spot alternative names like 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene, 3,5-difluoro-4-(trifluoromethoxy)bromobenzene, or even their CAS-number references. Each supplier likes to stick their own product code onto the bottle, each with a slight twist on the naming system. For anyone trying to order the right chemical or search literature, confirming the CAS number (214759-20-9) often feels like the only way to bypass the potential confusion.
Safety & Operational Standards
You don’t pick up or pour this compound with bare hands. Halogenated organics demand gloves, goggles, and, ideally, a fume hood. Vapors don’t shout their presence with a strong odor, but inhaling them brings risks—ranging from minor irritation to systemic toxicity in case of high exposure. Liquid splashes sting skin and eyes. Clean spills fast, store the bottle away from open flames, and avoid stashing it near oxidizers or strong acids. Following GHS labeling, you see the warning icons pop up on the bottle. Disposal swims in legal paperwork—environmental agencies keep an eagle eye on halogenated organics due to their persistent and bioaccumulative properties. In the lab, you count on dedicated halogenated-solvent waste drums and licensed disposal firms. Cheating on these protocols brings fines, endangers people, and haunts your conscience.
Application Area
Although not famous enough for mass production, this compound appears everywhere that researchers customize pharmaceutical leads, agrochemicals, and specialty materials. Fluorine-rich aromatics show up in cancer research, central nervous system agents, and fungicides chasing novel mechanisms. I’ve seen entire screens of kinase inhibitors or anti-inflammatory drugs start with a panel of fluorinated and brominated fragments, sometimes with the trifluoromethoxy group as a top pick for boosting metabolic stability or fine-tuning activity. Material scientists looking to achieve low dielectric constants or high performance in organic electronics have flirted with similar building blocks. Seldom is the molecule the final star; instead, it unlocks structure-activity relationships across whole libraries, nudging discovery projects forward.
Research & Development
Synthetic organic chemistry grows through both serendipity and dogged persistence. New cross-coupling methodologies mean compounds like this one became easier to use even as academic groups pushed the boundaries of what modifies aromatic rings. In industry, R&D teams treat these building blocks as test beds for computational modeling, where the effect of each substituent is explored before wet chemistry gets the green light. Automation and high-throughput experimentation now let a single chemist screen dozens of derivatives within a week—something unimaginable just two decades ago. In my own work, I’ve found the biggest hurdles weren’t finding new chemistry, but scaling up from bench to pilot plant without losing yield or purity to side reactions. Sharing data on routes, yields, and unexpected reactivity unlocks progress for everyone, not just the group holding the patent.
Toxicity Research
Worrying about toxicity isn't paranoia; it’s smart chemistry. Brominated and fluorinated aromatics demand a close look, as both classes infect the headlines from time to time—think about the legacy of PCBs and PFAS. Current data on this specific compound stays limited, but a careful reading of similar molecules suggests it can cause organ toxicity at high concentrations. Animal studies on close relatives show issues with bioaccumulation and moderate acute toxicity. Chronic exposure raises more flags, partly because these molecules stick around in the body and environment. That means every new application warrants not just efficacy testing, but rigorous in vitro and in vivo toxicity screenings—cytotoxicity, mutagenicity, and metabolism studies rank high on the agenda for anyone pushing forward with human or animal trials. In regulatory compliance, erring on the side of caution isn’t just ethical, it’s required, especially with regulators eager to keep persistent halogenated compounds in check.
Future Prospects
Looking out five and ten years, the market for specialty aryl halides and fluorinated aromatics keeps growing. As personalized medicine digs deeper into custom molecules, demand for flexible, tunable building blocks rises. Green chemistry initiatives will push the chemical community to discover less toxic, more efficient preparation methods that minimize hazardous waste and avoid persistent by-products. I expect machine learning-driven synthesis planning, AI-guided structure-activity predictions, and more transparent sharing of safety data to all play a role in making compounds like this safer and more useful. As science doubles down on sustainability, you’ll see shifting attitudes toward sourcing precursors and disposal, while simultaneously seeing innovation in fields from pharmaceuticals to polymers. A handful of challenges linger—mainly toxicity and environmental impact—but with the right blend of rigor and creativity, the toolkit of synthetic chemistry stands set to both manage risk and unlock new possibilities.
A Benzene Ring with a Twisted Personality
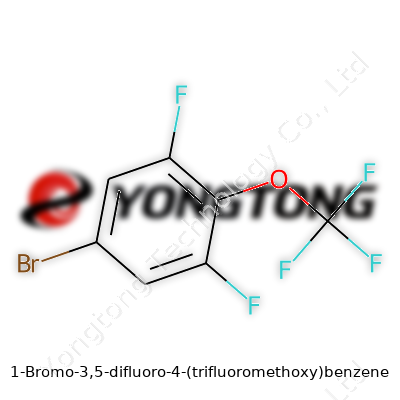
First glance at the name “1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene” might stir up anxiety in any chemistry class dropout, but this sort of compound shows up more often in the world than many suspect. Looking beyond the alphabet soup, the molecule brings together a well-known aromatic ring and a collection of halogen and ether groups that remind us of the way chemists pull strings to tailor material properties.
A benzene ring, six carbons in a neat hexagon with alternating double bonds, forms the skeleton. On it, a bromine atom takes up the first carbon spot in the ring. Bromine, big and heavy, brings bulk and reactivity to the molecule. Two fluorine atoms land on the third and fifth positions, across from each other. Fluorine’s electronegativity pulls electrons away and splits up the ring’s distribution of charge, which can tweak everything from how the molecule fits into a receptor to how it performs in electronics or as a building block in drug research.
Over on the fourth carbon, the story changes with an OCF3 group, called a trifluoromethoxy. Instead of just a plain oxygen or a simple fluorine, this feature brings three more fluorines attached to a single carbon, linked through that oxygen anchor. That’s no accident; trifluoromethoxy groups often pop up in medicinal chemistry or materials design, since they block metabolism in living systems and boost chemical stability. It’s not just about sticking more fluorine onto a ring—OCF3 disrupts the symmetry, changes electron clouds, and makes the molecule more lipophilic. That means it moves through oily environments (like cell membranes or plastic) with less friction, broadening its possible uses.
Fluorine’s Superpowers and the Industry’s Choices
After years in a research lab, I got used to seeing these sorts of halogenated aromatics come up in synthetic routes. Every substituent, like bromine or fluorine, brings a reason for being there. Fluorine’s small size and vast electron greediness can make a compound last longer in the body or tune its electronic characteristics for sensors. Bromine, on the other hand, acts as a handle for further modifications through cross-coupling reactions. A bromo position on a benzene ring allows chemists to swap in new groups, using palladium-catalyzed chemistry like Suzuki or Buchwald-Hartwig reactions.
Those choices, baked into a compound’s structure, come from direct ask-and-answer problems in chemistry. If a researcher wants a molecule to hang around longer—or wants to block enzymes from chewing up a drug—slapping on some fluorines often works. If the next synthesis step calls for a leaving group, bromine performs admirably.
The Risks and the Road Forward
Compounds like 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene don’t arrive without baggage. Fluorinated aromatics persist in the environment, raising issues in water and soil. The very stability chemists crave for drug development or high-performance plastics turns into an environmental hurdle. Once released, these molecules break down painfully slowly. That persistence brings potential toxicity or bioaccumulation, which cannot be swept aside.
Tackling these risks calls for real curiosity and honest work. Researchers are testing new ways to strip fluorinated compounds from water or degrade them with sunlight or specialized bacteria. Biochemists and policy makers also press start on making safer alternatives, using green chemistry principles to tune molecules for rapid breakdown after their job finishes. In my own work, any new halogenated material comes under scrutiny, balancing gain in performance with the legacy it leaves behind. As applications grow in electronics, pharmaceuticals, and materials science, responsibility and innovation have to keep pace with creativity at the bench and on the drawing board.
Medicinal Benefits and Healthcare Importance
My years spent working alongside pharmacists showed me how a single compound can change lives. Aspirin offers a solid example. For more than a century, people have relied on it to ease headaches and fevers and to lower inflammation. You’d be surprised how often doctors suggest it as a blood thinner, helping prevent strokes and heart attacks. The science lines up — research from the American Heart Association points to its everyday impact in reducing the risk of certain heart conditions.
In hospitals, sodium chloride plays a quiet but steady role. Nurses use saline solutions daily to clean wounds, hydrate patients, and carry medicines through IV drips. Without this compound, a lot of basic care just wouldn’t happen.
Modern Solutions in Agriculture
Farmers turn to fertilizers like ammonium nitrate to help crops reach their potential. In my early job on a family farm, I watched my neighbors choose fertilizers to fight poor soil and unpredictable yields. A paper from the University of Illinois reminds us these nitrogen-rich products help feed millions. Critics raise safety concerns, especially after accidents linked to storage. Tracking supply and improving oversight might limit dangers while letting growers keep their vital edge.
Everyday Industrial Workhorses
You probably bump into sulfuric acid every day without knowing it. This compound powers batteries, treats metals, and serves as a key ingredient in cleaning products. I used to fix cars, and I saw just how often battery acid keeps engines running. The chemical industry leans on sulfuric acid for making paints, soaps, and even the denim in blue jeans. According to the U.S. Geological Survey, demand for this acid reflects the overall strength of industry itself.
Safe Food and Clean Homes
Preservatives like sodium benzoate keep food fresh throughout its journey from factories to pantries. On the grocery shelf, you’ll spot it in sodas and jams. Food safety experts at the FDA monitor these additives to balance shelf life and consumer health. At home, bleach — mostly sodium hypochlorite — wipes out germs on kitchen counters and bathroom tiles. Using it safely prevents illness and earns the trust of families counting on clean spaces.
Tech Advancements Fueled by Chemistry
Silicon dioxide opens doors for engineers designing everything from smartphones to solar panels. My cousin, who works in semiconductor manufacturing, says they’d be lost without pure forms of this compound to build microchips. The tech world moves fast, but these building blocks never lose their worth.
Innovation has spurred new applications. Lithium carbonate has become crucial in rechargeable batteries for electric vehicles. Tesla and other companies invest heavily to secure these resources. The energy transition depends on the steady supply of foundational compounds that can store power for the long haul.
Finding the Right Balance
People benefit from these compounds every hour of every day. Oversight, transparency, and honesty from researchers help everyone use them with fewer risks. As science evolves, regular reviews on safety, storage, and sustainability can protect both health and the environment. We owe a lot to the simple compounds that keep daily life moving forward.
Looking Beyond the Product Label
Anyone who’s ever worked in manufacturing, science, or even a small workshop learns pretty quickly that the purity of a material changes everything. High-purity means peace of mind and predictable outcomes. In my own days trying to troubleshoot why a reaction didn’t hit the yield the textbook promised, I learned that even tiny impurities can throw things off. Picture trying to make a loaf of bread, but your flour has extra salt mixed in—it rarely ends well.
In the industrial world, purity isn’t just a technical spec—it has real impact on safety, on results, on costs. For pharmaceuticals, food production, or electronics, even one percent contaminant may make a world of difference, sometimes costing a company millions or risking health. Purity levels like 98%, 99%, and, for the pickiest applications, even 99.999%—each one serves a purpose, driven by its end use. Every step closer to total purity takes more time, technology, and cash, but for good reason. Medical labs, for example, can’t risk contaminated reagents screwing up clinical results. I’ve seen researchers send entire batches for revalidation only to find out the culprit sat in the original raw material’s specs.
The Question of Packaging Size
Let’s talk packaging, too. Imagine running a small-scale lab. You probably cringe at the idea of being forced to buy supplies in industrial drums that will sit around and soak up moisture or get contaminated before you’re halfway through. On the flip side, a manufacturer running processes around the clock will burn through tiny bottles in hours, racking up waste and cost.
Packaging size speaks to the scale of operation, shelf life, and even safety. Smaller bottles—sometimes down to grams—work for researchers, quality control labs, and startups that don’t need a warehouse’s worth of anything. Bulk packaging, on the other hand—25 kg bags, 200-liter drums, or IBC totes—play better with big budgets, steady demand, and automated lines. In fields like food processing, multiple packaging tiers handle everything from trial runs right up to full-scale production lines. During my stint at a chemical plant, the headaches caused by packaging mix-ups taught me to never ignore the details: wrong size order and suddenly the mixing bay sits idle, or frustrated staff spend hours ‘decanting’ product by hand.
Making Choices with Knowledge
It’s easy to gloss over an order form and just tick a box, but these decisions ripple out. A supplier’s transparency about purity, with methods like certificate of analysis or batch tracking, lets a buyer trust the numbers. Without these basics, teams fly blind. Sometimes, I’ve had to request extra documentation or even independent lab testing. In every case, a little extra time spent upfront prevented far bigger headaches later.
Toward Better Solutions
What helps most is clear information from suppliers: straightforward technical sheets, batch-level tracking, and honest answers when buyers ask. Customers benefit from flexible choices—more sizes, more transparency, and faster communication. As industries push for more responsible sourcing and less waste, the future probably holds more refillable and recyclable options, adding another layer of value for everyone involved. Decisions about purity and packaging may sound small, but in practice, these details speak volumes.
Understanding the Risks
Working with specialty chemicals like 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene really sharpens your sense of respect for proper lab practices. This compound brings a mix of halogens and an aromatic ring, all wrapped up in a small, reactive molecule. If you’ve ever smelled a strong aromatic compound that tingles at the back of your throat, you probably understand why it deserves careful attention. Many laboratory accidents arise because someone underestimated the risk or got careless while transferring such chemicals.
The Basics: Keep It Cool, Keep It Dry
Moisture and heat don’t play nicely with reactive organics. For this compound, the most important habit is finding a storage spot that remains cool and very dry. Seals on glass bottles make all the difference. Even a tiny crack in that seal can let in humidity and start a slow, ugly breakdown—slow enough that weeks or months later, you discover a ruined sample.
From my own experience, a dedicated chemical refrigerator (not the same one you use for food or biological samples) removes a lot of doubt. Chemicals like these won’t last long on a bench exposed to heat, light, and air.
Solid Containers and Safety Barriers
Avoid plastic bottles here, especially for long-term storage. Glass wins the reliability battle over time, especially with reactive or corrosive reagents. Over the years, I’ve seen certain solvents eat through plastic unexpectedly, which never ends well. For extra confidence, many labs double-bag the primary container with a sealed secondary bag and label every layer with hazard warnings. Spills from aromatic halides have a way of lingering in concrete and on gloves, so every extra precaution pays off later.
Labeling and Segregation Make a Difference
Nobody wants to sort through dozens of colorless liquids, wondering what could hurt them without a clear label. Every time we relabel new bottles, we add the chemical name, the date received or opened, and a hazard symbol. This simple act has prevented a lot of mishaps. Neighbors matter in storage, too—keep halogenated organics away from acids, strong bases, or oxidizers. Once, a neighboring student shelved peroxides next to halogen aromatic compounds, and we narrowly missed a more serious accident after an unnoticed leak.
Ventilation and Personal Protection
Nobody enjoys the smell of brominated aromatics in the morning. Always open bottles or prepare solutions in a good, functioning chemical fume hood. My personal rule is to treat this compound like any other potent halogenated aromatic—eye protection, thick nitrile gloves, a buttoned lab coat, and a clear working area. Even a small splash can burn skin or eyes, and inhaling those vapors will have you coughing your way out of the building.
Emergency Readiness
It pays to know where your spill kits and eyewash stations sit. Treat every use of compounds like this as a small drill for bigger emergencies. Every chemist has a spill story; you want yours to be about quick, effective cleanup, not costly mistakes. We talk through our protocol before running reactions, just to prevent confusion if something goes wrong.
Proactive cleanup and safe habits also limit unnecessary waste. If a bottle gets contaminated or looks off, it goes for safe disposal—never risk it by “trying it out.”
The Right Attitude
Keeping 1-Bromo-3,5-difluoro-4-(trifluoromethoxy)benzene safe isn’t about elaborate systems; it’s about steady habits, clear labels, and respect for your tools. Most incidents start with shortcuts or ignoring a small hazard; build good routines, and the compounds stay safe right alongside you.
A Closer Look at Chemical Hazards
Most people bump into chemicals every day, whether it’s through cleaning products, fuel, or the odd ingredient with a long name in a food package. I used to think a label like “irritant” or “flammable” was enough to get the picture, but my background in environmental work taught me the real story goes further. Chemicals, even those we keep under the kitchen sink, demand attention—not just for what they do, but what they can cause if ignored or mishandled.
Real Risks, Not Theoretical Ones
You don’t have to be a scientist to get why some caution matters. Bleach and ammonia make a cloud of toxic gas if mixed. Acetone in nail polish remover evaporates fast and can cause headaches. Acids don’t care if you’re careful; a single splash could damage skin for good. Data from the American Association of Poison Control Centers points out thousands of accidental chemical exposures every year, many in homes, not factories. So chemical safety is personal.
Labels and Data Sheets: Beyond the Fine Print
In my graduate lab days, we had a rule: never open a bottle before reading its Safety Data Sheet (SDS). The SDS lays it out—how it can get in your body, what harm it brings, and what to do if something spills. If you ask Google, “Is this chemical dangerous?” you might get a warning about burns or that it eats through metal. But you really learn something in the small print: some cause cancer in animals, others only harm when inhaled in dust form. The hazard changes with how you use the chemical.
Protecting Yourself and Others
The best shield is often a pair of gloves or a splash-proof apron. In the lab, goggles were non-negotiable, and at home, some chemicals should never hit bare hands. Always read the label and follow the instructions. It sounds obvious, but I’ve watched people ignore that “Use in a well-ventilated area” line and end up dizzy. Regular accidents with cleaning agents often stem from mixing, storing in the wrong containers, or skipping the ventilation step. If you look up OSHA reports, a big chunk of chemical injuries comes down to these small mishaps.
Environmental Consequences
It’s not just our bodies at stake. Dumping leftover chemicals in drains sends toxins right into water supplies. I’ve seen community gardens damaged by backyard pesticide use because someone dumped “harmless” leftovers. Experts from the EPA warn that trace amounts affect rivers and wildlife, building up over time. Chemicals in the wrong place don’t just vanish—they stick around, travel, and cause trouble we don’t always see right away.
Safer Paths Forward
Staying safe calls for practical steps. Keep chemicals in their original packaging. Store them away from kids and pets. Never combine products without checking safety advice. Many towns run hazardous waste collections; use them for disposal instead of pouring stuff down the sink. Push for strong laws that require clear warning labels. I support more education on chemical dangers in schools and at work. If people know what to look out for, they act smarter around risky stuff.
Everyday Choices Matter
Most accidents can be dodged just by reading instructions and respecting hazard signs. Looking up an SDS shouldn’t feel like overkill; it’s a basic part of handling chemicals safely. Years working around hazardous materials taught me that treating every chemical with care isn’t alarmist—it’s just smart, for your health and the planet’s.