PTSA (p-toluenesulfonic acid) acts as a strong acid catalyst in various organic reactions by protonating substrates, making them more electrophilic and susceptible to nucleophilic attack.
In esterification reactions, the mechanism involves the following steps[1, 2]:
- PTSA protonates the carbonyl oxygen of the carboxylic acid, activating it for nucleophilic addition.
- The alcohol nucleophilically attacks the activated carbonyl carbon, forming a tetrahedral intermediate.
- Proton transfer from the oxonium ion to the hydroxyl group of the tetrahedral intermediate occurs.
- The protonated hydroxyl group leaves as water, forming the protonated ester.
- Deprotonation of the ester yields the final ester product.
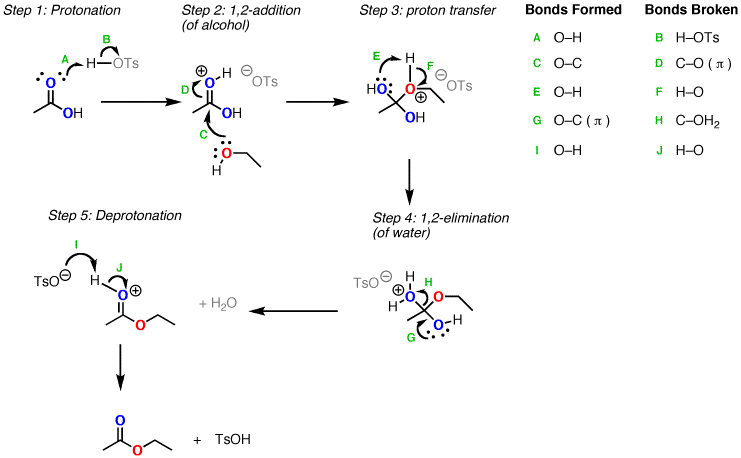
Each step in the esterification mechanism is reversible, so the reaction is an equilibrium. Excess alcohol or carboxylic acid is often used to drive the equilibrium forward. Water can also be removed to shift the equilibrium toward ester formation.
In acetalization reactions, the mechanism catalyzed by PTSA involves[2]:
- PTSA protonates the carbonyl group of the aldehyde or ketone, making it more electrophilic.
- Nucleophilic addition of one alcohol molecule to the activated carbonyl forms a hemiacetal intermediate.
- PTSA protonates the hydroxyl group of the hemiacetal, creating a good leaving group.
- A second alcohol molecule acts as a nucleophile, displacing water and forming the protonated acetal.
- Loss of a proton yields the final acetal product.
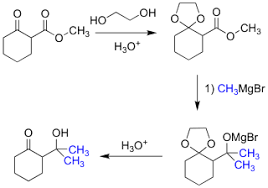 [3]
[3]
The acetalization reaction is also an equilibrium. Removal of water, often through a drying agent or distillation, drives the equilibrium toward acetal formation.
The strong acidity of PTSA enables it to protonate carbonyl groups and other substrates, increasing their electrophilicity. This facilitates nucleophilic addition reactions like esterification and acetalization. The reversibility of the individual steps means reaction conditions can be adjusted to favor product formation. PTSA’s solubility in organic solvents further enhances its utility as an acid catalyst for a wide range of synthetic transformations.
- David T. Macpherson, H.K.R., in Comprehensive Organic Functional Group Transformations, 1995, Comprehensive Organic Functional Group Transformations.1995.
- Eight-membered and larger Heterocyclic Rings and their Fused Derivatives, O.S.-m.R. and P.D. G. Cirrincione, in Comprehensive Heterocyclic Chemistry III, 2008, Eight-membered and larger Heterocyclic Rings and their Fused Derivatives, Other Seven-membered Rings.2008.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/19%3A_Aldehydes_and_Ketones-_Nucleophilic_Addition_Reactions/19.10%3A_Nucleophilic_Addition_of_Alcohols_-_Acetal_Formation.
