Abstract: P-tert-butyl toluene PTBT as the raw material, Oxygen as the oxidant, Glacial Acetate acid as the solvent, and Cobalt acetate as the catalyst. Studying the factors influencing P-tert-butyl benzoic acid (PTBBA) synthesis and fixing the best conditions for this process occurred on: the temperature is 90-95℃,the ratio between raw material and solvent is 1:4(m:m), the concentrate of catalyst is w=0.1-0.2, P-tert-butyl toluene(PTBT)’s conversion rate could up to 100% and the yield is 87%.
Key wards: P-tert-butyl toluene(PTBT) P-Tert-Butyl Benzoic Acid(PTBBA) Oxidization Synthesize
P-Tert-Butyl Benzoic Acid(PTBBA) is colorless needle crystal or crystalline powder, derivative of benzoic acid, and a important organic synthesis intermediate. Its main purposes are: (1) Used as modifier for alkyd resin production; (2) Used as additives for cutting oils and lubricants; (3) Used as nucleating agent of polypropylene; (4) Its barium, sodium, zinc salts could be used as stabilizer of polyvinyl chloride; (5) Used as food additives; (6) Used as regulator for polyester polymerization reaction and so on.
In 1930s, this product in laboratory research begun. Currently, there are product launching in America, Japan, Germany and other countries markets. However, our domestic production was not well developed, making home consumption of this product, especially the polyvinyl chloride and polypropylene industry, rely on import. Therefore, study and development on P-Tert-Butyl Benzoic Acid(PTBBA) has broad prospects.
Nitric acid oxidation method
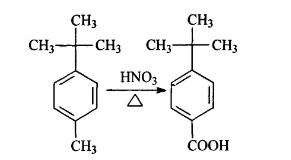
Preparation of crude PTBBA: Adding 26mL (0.2mol,29.6g) p-tert-butyl toluene (PTBT), 26mL 68%(0.4mol,37g) Nitric acid, and then 215mL water into the 500 mL autoclave. Closing the lid and starting the stirrer. The temperature is heated to 180℃ and the traction time is kept to be 8 h. After water cooling off, the cover was removed. Majority of the solids deposited at the autoclave bottom, and some was attaching to the cooling pipes. Collecting the solid, filtrating and drying, the weight is 34.5 g and productivity is 95.5%.
Purifying P-tert-butyl benzoic acid: Weighting 10g Sodium Hydroxide and making it into 10% solution with 90 mL water. Adding above solid into the solution. After completely dissolved, filtrating it. Adjusting the filtrate’s ph value to be around 3 with Hydrochloric acid. Then there is large quantity of crystalline happening. Stewing and filtrating, 33.4g material will be yielded and the productivity is 98.2%.
Even if the productivity is high with Nitric acid oxidation method to prepare P-tert-butyl benzoic acid, the causticity of nitric acid to the devices is great. During the reaction, the side products, such as nitrogen oxides, have great effect on the environment.
Acetate solvent catalytic oxidation method:
This method takes p-tert-butyl toluene (PTBT) as raw material, Cobalt Acetate as the catalyst and acetate acid as solvent. 250 mL stainless steel autoclave is the main reaction device, with mechanical stirrer. The air inlet and products outlet are at the bottom of the autoclave. The reaction is free redical chain reaction. The theory is below:
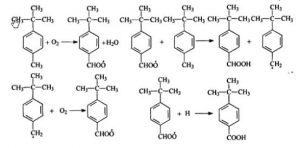
High temperature make p-tert-butyl toluene (PTBT) oxidized to yield P-tert-butyl benzoic acid with suitable catalyst. The productivity is high, but there is too many side products and the reaction takes too long time.
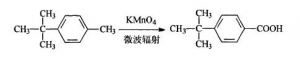
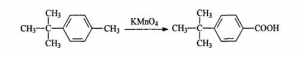
Microwave Radiation Method: The procedures are: adding 6 mL p-tert-butyl toluene, 8.4g Potassium Permanganate, 1.0g Benzyl triethyl ammonium chloride and 100mL water into 250 mL three-necked flash one by one. Shaking up and put it into the microwave reactor, assembling motor stirrer, reflux condenser and thermograph. Adjusting the microwave radiation power at 660W and keeping radiating for 50 min. After the reaction finished, pumping and filtering away Manganese Dioxide and get the remained solution concentrated and cooled to room temperature. Adding Hydrochloric acid to adjust the PH to be 2-3, cooling the solution till there is some crystalline come out. That is crude P-tert-butyl Benzoic acid (PTBBA). The fined products should be recrystallized with Toluene, the weight is 5.6g. And it is white needle crystalline. The final productivity is 82.6%. Melting point is 164-166℃.
Potassium Permanganate Oxidation Method:
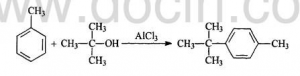
The procedures are: adding Toluene dried by Calcium Chloride anhydrous, and 30g Aluminium Trichloride powder into the 250mL three-necked flash with reflux condenser and motor stirrer. Splashing 40g dried tert-butyl alcohol into the flash slowly. Transferring the solution into 500 mL beaker and adding about 200g crushed ice. Layering and washing it with salt solution. Reducing the press and remove the remained Toluene.
Adding 300mL deionized water and 54.5g p-tert-butyl toluene into the 500mL three-necked flash, which is connected with reflux condenser and motor stirrer. While heating and stirring rapidly, adding Potassium Permanganate in lots. Keeping the reaction conducting 7-8 h till the purple solution doesn’t fade. Putting the solution into 500mL beaker and keeping it for one night. After filtering Manganese Dioxide away , adding hydrochloric acid. Repeating above procedures till the color turns into colorless and PH valued gets to be below 5. After washing, adding 100mL toluene white solid to be dissolved. The upper layer was reduced press to recover toluene. Cooling to obtain needle crystalline P-Tert-Butyl Benzoic Acid(PTBBA).
In the series reports of studies on synthesizing P-tert-butyl Benzoic acid (PTBBA), the methods, such as Microwave Radiation Method, Potassium Permanganate Oxidation Method, Nitric acid oxidation method, Acetate solvent catalytic oxidation method and the Solvent-free liquid phase oxidation method, have been introduced in details. Besides, there some other methods, like high-temperature gas-phase oxidation method. While being catalyzed, p-tert-butyl toluene is gasified and oxidized by the high temperature and yield Para-tert-butyl Benzoic acid (PTBBA). The electronic oxidization method is also workable in P-tert-butyl Benzoic acid’s preparation.
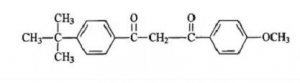
Application and Development Prospects of P-tert-butyl Benzoic acid (PTBBA): Sunscreens is the core of sunscreen cosmetics formulations. In 1928, USA released the first report on sunscreens all over the world. Till now, there are already forty kinds of sunscreens being developed. According to the function, they are divided into UV absorbers and Ultraviolet bulk toners. In 1997, Pursol 1789 was approved by USA FDI as safe and effective sunscreen. It is the most effective one among the rare kinds of UVA type absorbers. The structure is below:
With the wide use of polyvinyl chloride (PVC) in industry, agriculture, health care, daily necessities and other fields, the PVA heat stabilizer P-tert-butyl Benzoic acid (PTBBA)’s consumption is rising year by year. At present, many domestic PVC stabilizer producers are using P-tert-butyl Benzoic acid, mainly the liquid type. Korea, Japan, Mexico, Europe, America, India, Netherlands and other countries’ firms are also using it with large quantity. In addition, the market demand as lubricants adhesives and dye auxiliaries is also getting larger. Currently, America, Japan, Germany and other countries have developed P-tert-butyl Benzoic acid (PTBBA) production technique and have products available for the market. In the international market, only the France and Japan’s output has reached 18000 tons. However, our country’s export was only 300 ton last year. It is obvious that this product not only has large share for export, but the domestic demand is large. From the domestic and international market demand, the P-tert-butyl Benzoic acid (PTBBA) still has a huge shortfall in supply. So far, the P-tert-butyl Benzoic acid process synthetic route is not perfect , efficient , energy-saving , environmentally – friendly production process still has a practical application of the value .
